Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity
- PMID: 32773600
- PMCID: PMC7808363
- DOI: 10.1097/j.pain.0000000000002033
Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity
Abstract
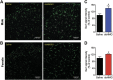
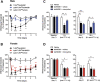
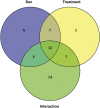
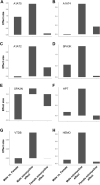
High mobility group box 1 protein (HMGB1) is increasingly regarded as an important player in the spinal regulation of chronic pain. Although it has been reported that HMGB1 induces spinal glial activation in a Toll-like receptor (TLR)4-dependent fashion, the aspect of sexual dimorphisms has not been thoroughly addressed. Here, we examined whether the action of TLR4-activating, partially reduced disulfide HMGB1 on microglia induces nociceptive behaviors in a sex-dependent manner. We found disulfide HMGB1 to equally increase microglial Iba1 immunoreactivity in lumbar spinal dorsal horn in male and female mice, but evoke higher cytokine and chemokine expression in primary microglial culture derived from males compared to females. Interestingly, TLR4 ablation in myeloid-derived cells, which include microglia, only protected male mice from developing HMGB1-induced mechanical hypersensitivity. Spinal administration of the glial inhibitor, minocycline, with disulfide HMGB1 also prevented pain-like behavior in male mice. To further explore sex difference, we examined the global spinal protein expression using liquid chromatography-mass spectrometry and found several antinociceptive and anti-inflammatory proteins to be upregulated in only male mice subjected to minocycline. One of the proteins elevated, alpha-1-antitrypsin, partially protected males but not females from developing HMGB1-induced pain. Targeting downstream proteins of alpha-1-antitrypsin failed to produce robust sex differences in pain-like behavior, suggesting that several proteins identified by liquid chromatography-mass spectrometry are required to modulate the effects. Taken together, the current study highlights the importance of mapping sex dimorphisms in pain mechanisms and point to processes potentially involved in the spinal antinociceptive effect of microglial inhibition in male mice.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


Similar articles
-
Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis.Pain. 2014 Sep;155(9):1802-1813. doi: 10.1016/j.pain.2014.06.007. Epub 2014 Jun 20. Pain. 2014. PMID: 24954167
-
Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain.Pain. 2021 Feb 1;162(2):459-470. doi: 10.1097/j.pain.0000000000002034. Pain. 2021. PMID: 32796317 Free PMC article.
-
Perineural high-mobility group box 1 induces mechanical hypersensitivity through activation of spinal microglia: Involvement of glutamate-NMDA receptor dependent mechanism in spinal dorsal horn.Biochem Pharmacol. 2021 Apr;186:114496. doi: 10.1016/j.bcp.2021.114496. Epub 2021 Mar 3. Biochem Pharmacol. 2021. PMID: 33667472
-
High mobility group box-1: A therapeutic target for analgesia and associated symptoms in chronic pain.Biochem Pharmacol. 2024 Apr;222:116058. doi: 10.1016/j.bcp.2024.116058. Epub 2024 Feb 15. Biochem Pharmacol. 2024. PMID: 38367818 Review.
-
Molecular mechanisms of lidocaine.Ann Med Surg (Lond). 2021 Aug 17;69:102733. doi: 10.1016/j.amsu.2021.102733. eCollection 2021 Sep. Ann Med Surg (Lond). 2021. PMID: 34457261 Free PMC article. Review.
Cited by
-
Mechanisms and treatments of neuropathic itch in a mouse model of lymphoma.J Clin Invest. 2023 Feb 15;133(4):e160807. doi: 10.1172/JCI160807. J Clin Invest. 2023. PMID: 36520531 Free PMC article.
-
Relationship Between Blood Cytokine Levels, Psychological Comorbidity, and Widespreadness of Pain in Chronic Pelvic Pain.Front Psychiatry. 2021 Jun 25;12:651083. doi: 10.3389/fpsyt.2021.651083. eCollection 2021. Front Psychiatry. 2021. PMID: 34248700 Free PMC article.
-
Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response.Brain Behav Immun. 2024 Feb;116:193-202. doi: 10.1016/j.bbi.2023.12.013. Epub 2023 Dec 9. Brain Behav Immun. 2024. PMID: 38081433 Free PMC article. Review.
-
Specialized pro-resolving mediator Maresin 1 attenuates pain in a mouse model of osteoarthritis.Osteoarthritis Cartilage. 2025 Mar;33(3):341-350. doi: 10.1016/j.joca.2024.10.018. Epub 2024 Nov 29. Osteoarthritis Cartilage. 2025. PMID: 39617202
-
Sex differences in depression: An immunological perspective.Brain Res Bull. 2023 May;196:34-45. doi: 10.1016/j.brainresbull.2023.02.016. Epub 2023 Feb 28. Brain Res Bull. 2023. PMID: 36863664 Free PMC article. Review.
References
-
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, Maruyama I. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 2005;115:1267–74. - PMC - PubMed
-
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. PAIN 2014;155:1802–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

