Characterizing the dynamic rheology in the pericellular region by human mesenchymal stem cell re-engineering in PEG-peptide hydrogel scaffolds
- PMID: 32773889
- PMCID: PMC7413226
- DOI: 10.1007/s00397-019-01142-2
Characterizing the dynamic rheology in the pericellular region by human mesenchymal stem cell re-engineering in PEG-peptide hydrogel scaffolds
Abstract
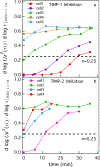
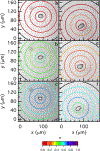
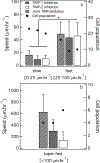
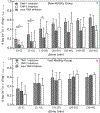
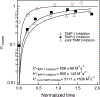
During wound healing, human mesenchymal stem cells (hMSCs) migrate to injuries to regulate inflammation and coordinate tissue regeneration. To enable migration, hMSCs re-engineer the extracellular matrix rheology. Our work determines the correlation between cell engineered rheology and motility. We encapsulate hMSCs in a cell-degradable peptide-polymeric hydrogel and characterize the change in rheological properties in the pericellular region using multiple particle tracking microrheology. Previous studies determined that pericellular rheology is correlated with motility. Additionally, hMSCs re-engineer their microenvironment by regulating cell-secreted enzyme, matrix metallopro-teinases (MMPs), activity by also secreting their inhibitors, tissue inhibitors of metalloproteinases (TIMPs). We independently inhibit TIMPs and measure two different degradation profiles, reaction-diffusion and reverse reaction-diffusion. These profiles are correlated with cell spreading, speed and motility type. We model scaffold degradation using Michaelis-Menten kinetics, finding a decrease in kinetics between joint and independent TIMP inhibition. hMSCs ability to regulate microenvironmental remodeling and motility could be exploited in design of new materials that deliver hMSCs to wounds to enhance healing.
Keywords: Michaelis-Menten kinetics; Multiple particle tracking microrheology; cellular degradation; matrix metalloproteinases; polymeric hydrogel scaffold; tissue inhibitor of metalloproteinases.
Figures

References
-
- abcam (2018) buffer and stock solutions for western blot. https://wwwabcamcom/protocols/buffer-and-stock-solutions-for-western-blot
-
- Adolf D, Martin JE (1990) Time-cure superposition during crosslinking. Macromolecules 23:3700–3704
-
- Bassi EJ, Candido de ALmeida D, Moraes-Vieira PMM, Camara NOS (2012) Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev and Rep 8:329–342 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
