Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression
- PMID: 32774054
- PMCID: PMC7383845
- DOI: 10.3748/wjg.v26.i26.3737
Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression
Abstract
Background: Immunotherapy targeting programmed death-1 (PD-1) or programmed death-ligand-1 (PD-L1) has been shown to be effective in a variety of malignancies but has poor efficacy in pancreatic ductal adenocarcinoma (PDAC). Studies have shown that PD-L1 expression in tumors is an important indicator of the efficacy of immunotherapy. Tumor cells usually evade chemotherapy and host immune surveillance by epigenetic changes. Protein arginine methylation is a common posttranslational modification. Protein arginine methyltransferase (PRMT) 1 is deregulated in a wide variety of cancer types, whose biological role in tumor immunity is undefined.
Aim: To investigate the combined effects and underlying mechanisms of anti-PD-L1 and type I PRMT inhibitor in pancreatic cancer in vivo.
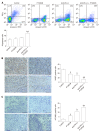
Methods: PT1001B is a novel type I PRMT inhibitor with strong activity and good selectivity. A mouse model of subcutaneous Panc02-derived tumors was used to evaluate drug efficacy, toxic and side effects, and tumor growth in vivo. By flow cytometry, we determined the expression of key immune checkpoint proteins, detected the apoptosis in tumor tissues, and analyzed the immune cells. Immunohistochemistry staining for cellular proliferation-associated nuclear protein Ki67, TUNEL assay, and PRMT1/PD-L1 immunofluorescence were used to elucidate the underlying molecular mechanism of the antitumor effect.
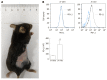
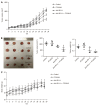
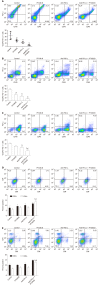
Results: Cultured Panc02 cells did not express PD-L1 in vitro, but tumor cells derived from Panc02 transplanted tumors expressed PD-L1. The therapeutic efficacy of anti-PD-L1 mAb was significantly enhanced by the addition of PT1001B as measured by tumor volume (1054.00 ± 61.37 mm3 vs 555.80 ± 74.42 mm3, P < 0.01) and tumor weight (0.83 ± 0.06 g vs 0.38 ± 0.02 g, P < 0.05). PT1001B improved antitumor immunity by inhibiting PD-L1 expression on tumor cells (32.74% ± 5.89% vs 17.95% ± 1.92%, P < 0.05). The combination therapy upregulated tumor-infiltrating CD8+ T lymphocytes (23.75% ± 3.20% vs 73.34% ± 4.35%, P < 0.01) and decreased PD-1+ leukocytes (35.77% ± 3.30% vs 6.48% ± 1.08%, P < 0.001) in tumor tissue compared to the control. In addition, PT1001B amplified the inhibitory effect of anti-PD-L1 on tumor cell proliferation and enhanced the induction of tumor cell apoptosis. PRMT1 downregulation was correlated with PD-L1 downregulation.
Conclusion: PT1001B enhances antitumor immunity and combining it with anti-PD-L1 checkpoint inhibitors provides a potential strategy to overcome anti-PD-L1 resistance in PDAC.
Keywords: Combination therapy; Pancreatic ductal adenocarcinoma; Programmed death-ligand-1 blockade; Protein arginine methyltransferase; Tumor microenvironment.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Figures
Similar articles
-
IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer.Gut. 2018 Feb;67(2):320-332. doi: 10.1136/gutjnl-2016-311585. Epub 2016 Oct 21. Gut. 2018. PMID: 27797936 Free PMC article.
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors.J Immunother Cancer. 2022 Mar;10(3):e003982. doi: 10.1136/jitc-2021-003982. J Immunother Cancer. 2022. PMID: 35260434 Free PMC article.
-
Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker.Cancer Med. 2020 Nov;9(21):8086-8121. doi: 10.1002/cam4.3410. Epub 2020 Sep 2. Cancer Med. 2020. PMID: 32875727 Free PMC article. Review.
-
PD-1/PD-L1 and immunotherapy for pancreatic cancer.Cancer Lett. 2017 Oct 28;407:57-65. doi: 10.1016/j.canlet.2017.08.006. Epub 2017 Aug 18. Cancer Lett. 2017. PMID: 28826722 Review.
Cited by
-
Histone modifiers at the crossroads of oncolytic and oncogenic viruses.Mol Ther. 2022 Jun 1;30(6):2153-2162. doi: 10.1016/j.ymthe.2022.02.006. Epub 2022 Feb 8. Mol Ther. 2022. PMID: 35143960 Free PMC article. Review.
-
Advances in pancreatic cancer epigenetics: From the mechanism to the clinic.World J Gastrointest Oncol. 2025 Jul 15;17(7):106238. doi: 10.4251/wjgo.v17.i7.106238. World J Gastrointest Oncol. 2025. PMID: 40697230 Free PMC article. Review.
-
PRMT1 Regulates EGFR and Wnt Signaling Pathways and Is a Promising Target for Combinatorial Treatment of Breast Cancer.Cancers (Basel). 2022 Jan 8;14(2):306. doi: 10.3390/cancers14020306. Cancers (Basel). 2022. PMID: 35053470 Free PMC article.
-
Research progress on PRMTs involved in epigenetic modification and tumour signalling pathway regulation (Review).Int J Oncol. 2023 May;62(5):62. doi: 10.3892/ijo.2023.5510. Epub 2023 Apr 7. Int J Oncol. 2023. PMID: 37026519 Free PMC article. Review.
-
Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies.Cells. 2021 Jan 11;10(1):124. doi: 10.3390/cells10010124. Cells. 2021. PMID: 33440687 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Collisson EA, Olive KP. Pancreatic Cancer: Progress and Challenges in a Rapidly Moving Field. Cancer Res. 2017;77:1060–1062. - PubMed
-
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

