Coronary Microcirculation in Aortic Stenosis: Pathophysiology, Invasive Assessment, and Future Directions
- PMID: 32774184
- PMCID: PMC7396014
- DOI: 10.1155/2020/4603169
Coronary Microcirculation in Aortic Stenosis: Pathophysiology, Invasive Assessment, and Future Directions
Abstract
With the increasing prevalence of aortic stenosis (AS) due to a growing elderly population, a proper understanding of its physiology is paramount to guide therapy and define severity. A better understanding of the microvasculature in AS could improve clinical care by predicting left ventricular remodeling or anticipate the interplay between epicardial stenosis and myocardial dysfunction. In this review, we combine five decades of literature regarding microvascular, coronary, and aortic valve physiology with emerging insights from newly developed invasive tools for quantifying microcirculatory function. Furthermore, we describe the coupling between microcirculation and epicardial stenosis, which is currently under investigation in several randomized trials enrolling subjects with concomitant AS and coronary disease. To clarify the physiology explained previously, we present two instructive cases with invasive pressure measurements quantifying coexisting valve and coronary stenoses. Finally, we pose open clinical and research questions whose answers would further expand our knowledge of microvascular dysfunction in AS. These trials were registered with NCT03042104, NCT03094143, and NCT02436655.
Copyright © 2020 Jo M. Zelis et al.
Conflict of interest statement
JMZ reports no support or industry relationships. PALT, NHJP, RLK, KLG, and NPJ have a patent pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology. PALT reports no additional support or industry relationships. NHPJ receives institutional grant support from Abbott, serves as a consultant for Abbott and Opsens, and possesses equity in Philips, GE, ASML, and Heartflow. BDB has received institutional research grants and consulting fees from Abbott Vascular (formerly St. Jude Medical), Boston Scientific, and Opsens. BDB, RLK, KLG, and NPJ have a patent pending on correcting pressure signals from fluid-filled catheters. RLK reports no additional support or industry relationships. KLG is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664 and K171303), software packages for cardiac positron emission tomography image processing, analysis, and absolute flow quantification. NPJ receives internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis, has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm (commercialized under 510(k) K191008), and has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE-FLOW, NCT02328820), studies using intracoronary pressure and flow sensors.
Figures

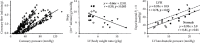
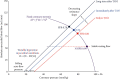



References
-
- Andersen H. R., Knudsen L. L., Hasenkam J. M. Transluminal implantation of artificial heart valves. description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. European Heart Journal. 1992;13(5):704–708. doi: 10.1093/oxfordjournals.eurheartj.a060238. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
