Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice
- PMID: 32774242
- PMCID: PMC7381385
- DOI: 10.3389/fncel.2020.00189
Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice
Abstract
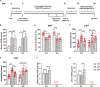
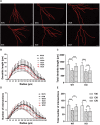
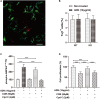
Adiponectin, a cytokine secreted by mature adipocytes, proves to be neuroprotective. We have previously reported that running triggers adiponectin up-regulation which subsequently promotes generation of hippocampal neurons and thereby alleviates depression-like behaviors in non-stressed mice. However, under the stressing condition, whether adiponectin could still exert antidepressant-like effects following exercise remained unexplored. In this study, by means of repeated corticosterone injections to mimic stress insult and voluntary wheel running as physical exercise intervention, we examined whether exercise-elicited antidepressive effects might involve adiponectin's regulation on hippocampal neurogenesis and dendritic plasticity in stressed mice. Here we show that repeated injections of corticosterone inhibited hippocampal neurogenesis and impaired dendritic morphology of neurons in the dentate gyrus of both wild-type and adiponectin-knockout mice comparably, which subsequently evoked depression-like behaviors. Voluntary wheel running attenuated corticosterone-suppressed neurogenesis and enhanced dendritic plasticity in the hippocampus, ultimately reducing depression-like behaviors in wild-type, but not adiponectin-knockout mice. We further demonstrate that such proneurogenic effects were potentially achieved through activation of the AMP-dependent kinase (AMPK) pathway. Our study provides the first evidence that adiponectin signaling is essential for physical exercise-triggered effects on stress-elicited depression by retaining the normal proliferation of neural progenitors and dendritic morphology of neurons in the hippocampal dentate gyrus, which may depend on activation of the AMPK pathway.
Keywords: adiponectin; dendritic plasticity; dentate gyrus; depression; hippocampal neurogenesis; voluntary exercise.
Copyright © 2020 Wang, Liang, Chen, Yau, Sun, Cheng, Xu, So and Li.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

