NOD2 Deficiency Promotes Intestinal CD4+ T Lymphocyte Imbalance, Metainflammation, and Aggravates Type 2 Diabetes in Murine Model
- PMID: 32774333
- PMCID: PMC7381387
- DOI: 10.3389/fimmu.2020.01265
NOD2 Deficiency Promotes Intestinal CD4+ T Lymphocyte Imbalance, Metainflammation, and Aggravates Type 2 Diabetes in Murine Model
Abstract
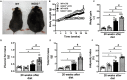
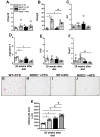
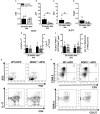
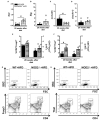
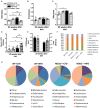
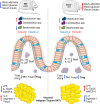
Type 2 diabetes (T2D) is a metabolic disease characterized by increased inflammation, NOD-like receptors (NLRs) activation and gut dysbiosis. Our research group has recently reported that intestinal Th17 response limits gut dysbiosis and LPS translocation to visceral adipose tissue (VAT), protecting against metabolic syndrome. However, whether NOD2 receptor contributes intestinal Th17 immunity, modulates dysbiosis-driven metabolic tissue inflammation, and obesity-induced T2D remain poorly understood. In this context, we observed that mice lacking NOD2 fed a high-fat diet (HFD) display severe obesity, exhibit greater adiposity, and more hepatic steatosis compared to HFD-fed wild-type (WT) mice. In addition, they develop increased hyperglycemia, worsening of glucose intolerance, and insulin resistance. Notably, the deficiency of NOD2 causes a deviation from M2 macrophage and regulatory T cells (Treg) to M1 macrophage and mast cells into VAT compared to WT mice fed HFD. An imbalance was also observed in Th17/Th1 cell populations, with reduced IL-17 and IL-22 gene expression in the mesenteric lymph nodes (MLNs) and ileum, respectively, of NOD2-deficient mice fed HFD. 16S rRNA sequencing indicates lower richness, alpha diversity, and a depletion of Allobaculum, Lactobacillus, and enrichment with Bacteroides genera in these mice compared to HFD-fed WT mice. These alterations were associated with disrupted tight-junctions expression, augmented serum LPS, and bacterial translocation into VAT. Overall, NOD2 activation is required for a protective Th17 over Th1 immunity in the gut, which seems to decrease gram-negative bacteria outgrowth in gut microbiota, attenuating the endotoxemia, metainflammation, and protecting against obesity-induced T2D.
Keywords: Innate immunity receptor; gut microbiota; helper T lymphocytes; metainflammation; obesity and type 2 diabetes.
Copyright © 2020 Carlos, Pérez, Leite, Rocha, Martins, Pereira, Fraga-Silva, Pucci, Ramos, Câmara, Bonato, Tostes and Silva.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

