Gaps and Doubts in Search to Recognize Glioblastoma Cellular Origin and Tumor Initiating Cells
- PMID: 32774372
- PMCID: PMC7396023
- DOI: 10.1155/2020/6783627
Gaps and Doubts in Search to Recognize Glioblastoma Cellular Origin and Tumor Initiating Cells
Abstract
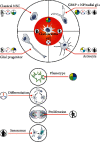
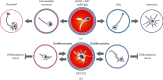
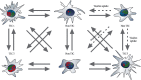
Cellular origin of glioblastoma (GB) is constantly discussed and remains a controversial subject. Unfortunately, neurobiologists are not consistent in defining neural stem cells (NSC) complicating this issue even further. Nevertheless, some suggestions referring to GB origin can be proposed based on comparing GB to central nervous system (CNS) cells. Firstly, GB cells show in vitro differentiation pattern similar to GFAP positive neural cells, rather than classical (GFAP negative) NSC. GB cells in primary cultures become senescent in vitro, similar to GFAP positive neural progenitors, whereas classical NSC proliferate in vitro infinitely. Classical NSC apoptosis triggered by introduction of IDH1R132H undermines hypothesis stating that IDH-mutant (secondary) GB origins from these NSC. Analysis of biological role of typical IDH-wildtype (primary) GB oncogene such as EGFRvIII also favors GFAP positive cells rather than classical NSC as source of GB. Single-cell NGS and single-cell transcriptomics also suggest that GFAP positive cells are GB origin. Considering the above-mentioned and other discussed in articles data, we suggest that GFAP positive cells (astrocytes, radial glia, or GFAP positive neural progenitors) are more likely to be source of GB than classical GFAP negative NSC, and further in vitro assays should be focused on these cells. It is highly possible that several populations of tumor initiating cells (TIC) exist within GB, adjusting their phenotype and even genotype to various environmental conditions including applied therapy and periodically going through different TIC states as well as non-TIC state. This adjustment is driven by changes in number and types of amplicons. The existence of various populations of TIC would enable creating neoplastic foci in different environments and increase tumor aggressiveness.
Copyright © 2020 Aneta Wlodarczyk et al.
Conflict of interest statement
The authors have declared that there are no conflicts of interest regarding the publication of this article.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

