Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis
- PMID: 32774474
- PMCID: PMC7394035
- DOI: 10.1177/1758835920940927
Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis
Abstract
Background: This network meta-analysis assessed the comparative risk of grade 3-5 and grade 5 treatment-related adverse events (TRAEs) for immune checkpoint inhibitors (ICIs), either alone or in combination with other modalities, for cancer treatment.
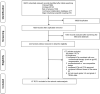
Methods: PubMed, Embase, Cochrane Library, Web of Science, and recent predominant oncology congresses were searched for relevant phase II and phase III randomized controlled trials (RCTs). As outcomes, grade 3-5, and grade 5 TRAE outcomes were reported as odds ratios and 95% confidence intervals.
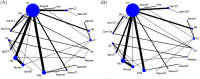
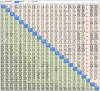
Results: In 67 RCTs involving 36,422 patients and 19 ICIs, the incidence of grade 3-5 and grade 5 TRAEs was 17.9% and 0.8% with ICI monotherapy and 46.3% and 1.4%, respectively, with combinatorial therapy. Pneumonitis was the most common cause of grade 5 TRAEs following either monotherapy (16.3%) or combinatorial therapy (11.4%). Regarding grade 3-5 TRAEs, atezolizumab + chemotherapy (CT) and antiangiogenic therapy (AT) (atezolizumab + CAT), pembrolizumab + CT, ipilimumab + CT, and atezolizumab + CT were more toxic than any ICI monotherapy, pembrolizumab or nivolumab + radiotherapy (RT), and ICIs dual therapy (durvalumab + tremelimumab and nivolumab + ipilimumab). Tremelimumab, ipilimumab, durvalumab, and pembrolizumab were, however, associated with higher grade 5 TRAEs than combinatorial treatments. Atezolizumab + CAT was the most toxic and nivolumab + RT was the least toxic of combinatorial treatments; among monotherapies, tremelimumab and avelumab were the most and least toxic, respectively. The toxicity ranking changed with type of grade 3-5 TRAEs.
Conclusions: Compared with combinatorial therapy, ICI monotherapy caused lower grade 3-5 TRAEs, but some monotherapies resulted in a higher incidence of fatal TRAEs. Atezolizumab + CAT and nivolumab + RT were the most and least toxic of combinatorial treatments, respectively, and tremelimumab and avelumab were the most and least toxic of the monotherapies, respectively.
Keywords: antiangiogenic therapy; chemotherapy; immune checkpoint inhibitor; network meta-analysis; radiotherapy; treatment-related adverse events.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther 2016; 100: 242–251. - PubMed
-
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. - PubMed
-
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017; 28: 2377–2385. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

