STAT3-PTTG11 abrogation inhibits proliferation and induces apoptosis in malignant glioma cells
- PMID: 32774480
- PMCID: PMC7405341
- DOI: 10.3892/ol.2020.11867
STAT3-PTTG11 abrogation inhibits proliferation and induces apoptosis in malignant glioma cells
Abstract
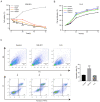
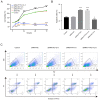
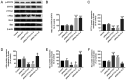
Pituitary tumor transforming gene 1 (PTTG11) is abundantly expressed in glioma. Our previous study demonstrated that the downregulation of PTTG11 gene expression significantly inhibited the proliferation, migration and invasion ability, and increased the apoptosis of SHG44 glioma cells. However, the molecular mechanisms that regulate PTTG11 and its actions remain elusive. In the present study, CCK-8 and flow cytometry assays were used to assess the proliferation/viability and apoptosis, respectively, of the human glioma U251 cell line. STAT3-PTTG1 signals were further evaluated by western blotting. The findings of the present study revealed that STAT3 induced PTTG11 expression, which subsequently induced downstream c-Myc and Bcl-2 expression while inhibiting Bax expression, thereby promoting cell viability and inhibiting apoptosis. PTTG11 suppression via siRNA inhibited the viability and increased the apoptosis of glioma cells induced by the STAT3 activator S3I-201. c-Myc and Bcl-2 expression was suppressed by PTTG11 inhibition. The findings of the present study suggest that the STAT3-PTTG11 signaling pathway may play an important role in glioma progression by regulating cell proliferation and apoptosis.
Keywords: STAT3; apoptosis; malignant glioma; pituitary tumor transforming gene 1; proliferation.
Copyright © 2020, Spandidos Publications.
Figures

References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Branger DF, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Navarro L, Gil-Benso R, Megías J, Muñoz-Hidalgo L, San- Miguel T, Callaghan RC, González-Darder JM, López-Ginés C, Cerdá-Nicolás MJ. Alteration of major vault protein in human glioblastoma and its relation with EGFR and PTEN status. Neuroscience. 2015;297:243–251. doi: 10.1016/j.neuroscience.2015.04.005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
