Involvement of MM cell-derived exosomes in T lymphocytes immune responses
- PMID: 32774504
- PMCID: PMC7405633
- DOI: 10.3892/ol.2020.11892
Involvement of MM cell-derived exosomes in T lymphocytes immune responses
Abstract
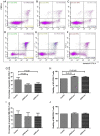
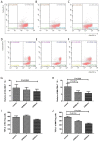
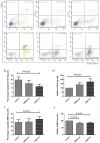
Exosomes were reported to mediate cell communication in the tumor microenvironment; however, the effects of multiple myeloma (MM)-derived exosomes on the quantity and function of T cells remain unknown. Exosomes were extracted from MM cell lines (OPM2 and U266B1) by ultracentrifugation using a Total Exosome Isolation kit. Exosomes were co-cultured with CD4+ T, CD8+ T and regulatory T (Treg) cells that were isolated from healthy donors (HDs) and patients with MM using magnetic beads. Flow cytometry was used to detect T cells apoptosis and expression of perforin and granzyme B in CD8+ T cells. Cell viability was detected using Cell Counting kit-8, and interleukin 10 (IL-10) and transforming growth factor β (TGF-β) in cell supernatants were detected by ELISA. The apoptosis of HD-CD4+ T was higher in the OPM2 group, and viability in the U266B1 group was decreased. The apoptosis of HD-CD8+ T decreased in the OPM2 and U266B1 groups, and cell viability increased in the OPM2 and the U266B1 groups. Perforin of HD-CD8+ T in the U266B1 group was lower while perforin of MM-CD8+ T in OPM2 and U266B1 groups was markedly decreased. The apoptosis of HD-Treg was lower in the U266B1 group, but apoptosis of MM-Treg was higher in the U266B1 group. The viability of HD-Treg in U266B1 group increased but the viability of MM-Treg in OPM2 and U266B1 groups decreased. TGF-β from MM-Treg decreased in the OPM2 and U266B1 groups when compared with the control group (P<0.05). MM-derived exosomes promote apoptosis and inhibit proliferation of HD-CD4+ T, inhibit apoptosis and promote proliferation, but inhibit perforin of HD-CD8+ T, inhibit apoptosis and promote proliferation HD-Treg, and inhibit perforin of MM-CD8+ T and TGF-β secretion of MM-Treg.
Keywords: T lymphocyte; exosomes; immunity; multiple myeloma.
Copyright: © Shao et al.
Figures


Similar articles
-
Impact of exosomes derived from adipose stem cells on lymphocyte proliferation and phenotype in mouse skin grafts.Extracell Vesicles Circ Nucl Acids. 2025 Mar 7;6(1):141-157. doi: 10.20517/evcna.2024.52. eCollection 2025. Extracell Vesicles Circ Nucl Acids. 2025. PMID: 40206795 Free PMC article.
-
Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients.Gastroenterology. 2007 Jun;132(7):2328-39. doi: 10.1053/j.gastro.2007.03.102. Epub 2007 Apr 14. Gastroenterology. 2007. PMID: 17570208
-
Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells.J Natl Cancer Inst. 2014 Dec 12;107(1):363. doi: 10.1093/jnci/dju363. Print 2015 Jan. J Natl Cancer Inst. 2014. PMID: 25505237
-
Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes).Biochem Soc Trans. 2013 Feb 1;41(1):245-51. doi: 10.1042/BST20120265. Biochem Soc Trans. 2013. PMID: 23356291 Free PMC article. Review.
-
Enhanced suppression of polyclonal CD8+25+ regulatory T cells via exosomal arming of antigen-specific peptide/MHC complexes.J Leukoc Biol. 2017 May;101(5):1221-1231. doi: 10.1189/jlb.3A0716-295RR. Epub 2017 Jan 17. J Leukoc Biol. 2017. PMID: 28096300
Cited by
-
Exosome as a crucial communicator between tumor microenvironment and gastric cancer (Review).Int J Oncol. 2024 Mar;64(3):28. doi: 10.3892/ijo.2024.5616. Epub 2024 Jan 19. Int J Oncol. 2024. PMID: 38240092 Free PMC article. Review.
-
What happens to regulatory T cells in multiple myeloma.Cell Death Discov. 2023 Dec 21;9(1):468. doi: 10.1038/s41420-023-01765-8. Cell Death Discov. 2023. PMID: 38129374 Free PMC article. Review.
-
Immune modulation by melanoma-derived exosomes: suppression of BALB/c mice splenic cell proliferation, induction of apoptosis, and cell cycle arrest.Mol Biol Rep. 2025 Aug 6;52(1):794. doi: 10.1007/s11033-025-10899-0. Mol Biol Rep. 2025. PMID: 40767919
-
Tumor-Derived Extracellular Vesicles in Cancer Immunoediting and Their Potential as Oncoimmunotherapeutics.Cancers (Basel). 2022 Dec 23;15(1):82. doi: 10.3390/cancers15010082. Cancers (Basel). 2022. PMID: 36612080 Free PMC article. Review.
-
Paediatric pre-B acute lymphoblastic leukaemia-derived exosomes regulate immune function in human T cells.J Cell Mol Med. 2022 Aug;26(16):4566-4576. doi: 10.1111/jcmm.17482. Epub 2022 Jul 13. J Cell Mol Med. 2022. PMID: 35822529 Free PMC article.
References
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
