Effect of microfluidic processing on the viability of boar and bull spermatozoa
- PMID: 32774586
- PMCID: PMC7402706
- DOI: 10.1063/5.0013919
Effect of microfluidic processing on the viability of boar and bull spermatozoa
Abstract
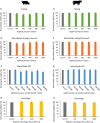
The use of microfluidics in artificial reproductive technologies for manipulation or assessment of spermatozoa is unique in the sense that it is not always an end point measurement and the sample may be used afterward. During microfluidic processing, spermatozoa are exposed to shear stress, which may harm viability and functioning of spermatozoa. The shear stresses during general microfluidic processing steps were calculated and compared to estimated shear stresses during ejaculation. The viability of boar and bull spermatozoa after microfluidic processing was studied and compared to the typical handling method (centrifugation) and to a control (the sample in a tube at the same temperature). The boar spermatozoa showed a small but significant decrease in viability of 6% after microfluidic handling. Bull spermatozoa proved to be less susceptible to shear stress and were not significantly affected by microfluidic processing. These data indicate that the impact of microfluidic processing on the viability of boar and bull spermatozoa is less than the literature values reported for flow cytometry and comparable to the impact of centrifugation.
© 2020 Author(s).
Figures

References
-
- Morrell J. M., Artificial Insemination in Farm Animals (InTech Open, 2011), p. 1.
-
- Godke R. A., Sansinena M., and Youngs C. R., Transgenic Animal Technology (Elsevier, 2014), p. 581.
LinkOut - more resources
Full Text Sources
