Blocking pannexin1 reduces airway inflammation in a murine model of asthma
- PMID: 32774761
- PMCID: PMC7407700
Blocking pannexin1 reduces airway inflammation in a murine model of asthma
Abstract
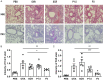
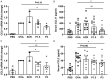
Stressed or injured cells release ATP into the extracellular milieu via the pannexin1 (Panx1) channels, which is the basis of inflammation in a variety of conditions, including allergic lung inflammation. Although the role of Panx1 in mediating inflammation has been well established, the role of its mimetic peptide, 10Panx1, which inhibits ATP release from Panx1 channels, in allergic asthma remains understudied. The aim of this study was to evaluate the effects of using 10Panx1 to inhibit Panx1 channel in a murine model of ovalbumin (OVA)-induced asthma. We demonstrate that blockade of Panx1 significantly attenuated goblet cell hyperplasia and inflammatory cell infiltration into the lungs of OVA-sensitized mice. Inhibition of Panx1 also reduced the total and eosinophil cell numbers in the bronchoalveolar lavage fluid (BALF) and reduced expression of CCL11 and CCL2 in lung tissues from mice. Moreover, we detected lower levels of IL-5 and IL-13 in the culture supernatant of OVA-restimulated splenocytes from 10Panx1-treated mice. Collectively, our findings suggest that Panx1 inhibition of allergen-mediated lung inflammation has the potential to suppress allergic responses in asthma.
Keywords: 10Panx1; EXtracellular ATP; asthma; chemokine; lung inflammation; pannexin1.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures



Similar articles
-
Blocking ATP-releasing channels prevents high extracellular ATP levels and airway hyperreactivity in an asthmatic mouse model.Am J Physiol Lung Cell Mol Physiol. 2021 Aug 1;321(2):L466-L476. doi: 10.1152/ajplung.00450.2020. Epub 2021 Jul 7. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34231389
-
MFG-E8/integrin β3 signaling contributes to airway inflammation response and airway remodeling in an ovalbumin-induced murine model of asthma.J Cell Biochem. 2018 Nov;119(11):8887-8896. doi: 10.1002/jcb.27142. Epub 2018 Aug 4. J Cell Biochem. 2018. PMID: 30076648
-
Administration of mycobacterial Ag85A and IL-17A fusion protein attenuates airway inflammation in a murine model of asthma.Int Immunopharmacol. 2013 Dec;17(4):1067-74. doi: 10.1016/j.intimp.2013.10.009. Int Immunopharmacol. 2013. PMID: 24455775
-
Pannexin1 channels-a potential therapeutic target in inflammation.Front Cell Dev Biol. 2022 Nov 9;10:1020826. doi: 10.3389/fcell.2022.1020826. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438559 Free PMC article. Review.
-
Molecular pathways of pannexin1-mediated neurotoxicity.Front Physiol. 2014 Feb 11;5:23. doi: 10.3389/fphys.2014.00023. eCollection 2014. Front Physiol. 2014. PMID: 24575045 Free PMC article. Review.
Cited by
-
Pharmacology of pannexin channels.Curr Opin Pharmacol. 2023 Apr;69:102359. doi: 10.1016/j.coph.2023.102359. Epub 2023 Feb 28. Curr Opin Pharmacol. 2023. PMID: 36858833 Free PMC article. Review.
-
Novel Naphthyridones Targeting Pannexin 1 for Colitis Management.Adv Sci (Weinh). 2025 Feb;12(7):e2411538. doi: 10.1002/advs.202411538. Epub 2024 Dec 30. Adv Sci (Weinh). 2025. PMID: 39739600 Free PMC article.
-
ATP functions as a primary alarmin in allergen-induced type 2 immunity.Am J Physiol Cell Physiol. 2023 Nov 1;325(5):C1369-C1386. doi: 10.1152/ajpcell.00370.2023. Epub 2023 Oct 16. Am J Physiol Cell Physiol. 2023. PMID: 37842751 Free PMC article. Review.
-
Proximal tubule pannexin 1 contributes to mitochondrial dysfunction and cell death during acute kidney injury.Am J Physiol Renal Physiol. 2025 Jun 1;328(6):F830-F849. doi: 10.1152/ajprenal.00226.2024. Epub 2025 Apr 17. Am J Physiol Renal Physiol. 2025. PMID: 40241514 Free PMC article.
-
Reducing Lung ATP Levels and Alleviating Asthmatic Airway Inflammation through Adeno-Associated Viral Vector-Mediated CD39 Expression.Biomedicines. 2021 Jun 8;9(6):656. doi: 10.3390/biomedicines9060656. Biomedicines. 2021. PMID: 34201190 Free PMC article.
References
-
- Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50:975–991. - PubMed
-
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. - PubMed
-
- Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. - PubMed
LinkOut - more resources
Full Text Sources
