POT1-TPP1 telomere length regulation and disease
- PMID: 32774788
- PMCID: PMC7385035
- DOI: 10.1016/j.csbj.2020.06.040
POT1-TPP1 telomere length regulation and disease
Abstract
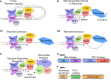
Telomeres are DNA repeats at the ends of linear chromosomes and are replicated by telomerase, a ribonucleoprotein reverse transcriptase. Telomere length regulation and chromosome end capping are essential for genome stability and are mediated primarily by the shelterin and CST complexes. POT1-TPP1, a subunit of shelterin, binds the telomeric overhang, suppresses ATR-dependent DNA damage response, and recruits telomerase to telomeres for DNA replication. POT1 localization to telomeres and chromosome end protection requires its interaction with TPP1. Therefore, the POT1-TPP1 complex is critical to telomere maintenance and full telomerase processivity. The aim of this mini-review is to summarize recent POT1-TPP1 structural studies and discuss how the complex contributes to telomere length regulation. In addition, we review how disruption of POT1-TPP1 function leads to human disease.
Keywords: ATM, Ataxia Telangiectasia Mutated protein; ATR, Ataxia Telangiectasia and Rad3-related Protein; CST, CTC1, Stn1 and Ten1; CTC1, Conserved Telomere Capping Protein 1; POT1; POT1, Protection of telomere 1; RAP1, Repressor/Activator Protein 1; RPA, Replication Protein A; SMCHD1, Structural Maintenance Of Chromosomes Flexible Hinge Domain Containing 1; Shelterin; Stn1, Suppressor of Cdc Thirteen; TERC, Telomerase RNA; TERT, Telomerase Reverse Transcriptase; TIN2, TRF1- and TRF2-Interacting Nuclear Protein 2; TPP1; TPP1 also known as ACD, Adrenocortical Dysplasia Protein Homolog; TRF1, Telomere Repeat binding Factor 1; TRF2, Telomere Repeat binding Factor 2; TSPYL5, Testis-specific Y-encoded-like protein 5; Telomerase; Telomeres; Ten1, Telomere Length Regulation Protein; USP7, ubiquitin-specific-processing protease 7.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Palm W., de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. Epub 2008/08/06. doi: 10.1146/annurev.genet.41.110306.130350. PubMed PMID: 18680434. - PubMed
-
- Cech T.R., Lingner J. Telomerase and the chromosome end replication problem. Ciba Found Symp. 1997;211:20–28. Epub 1997/01/01. doi: 10.1002/9780470515433.ch3. PubMed PMID: 9524749. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
