Expression of smooth muscle-like effectors and core cardiomyocyte regulators in the contractile papillae of Ciona
- PMID: 32774829
- PMCID: PMC7397655
- DOI: 10.1186/s13227-020-00162-x
Expression of smooth muscle-like effectors and core cardiomyocyte regulators in the contractile papillae of Ciona
Abstract
Background: The evolution of vertebrate smooth muscles is obscured by lack of identifiable smooth muscle-like cells in tunicates, the invertebrates most closely related to vertebrates. A recent evolutionary model was proposed in which smooth muscles arose before the last bilaterian common ancestor, and were later diversified, secondarily lost or modified in the branches leading to extant animal taxa. However, there is currently no data from tunicates to support this scenario.
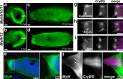
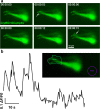
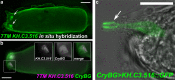
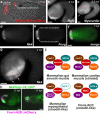
Methods and results: Here, we show that the axial columnar cells, a unique cell type in the adhesive larval papillae of the tunicate Ciona, are enriched for orthologs of vertebrate smooth/non-muscle-specific effectors of contractility, in addition to developing from progenitors that express conserved cardiomyocyte regulatory factors. We show that these cells contract during the retraction of the Ciona papillae during larval settlement and metamorphosis.
Conclusions: We propose that the axial columnar cells of Ciona are a myoepithelial cell type required for transducing external stimuli into mechanical forces that aid in the attachment of the motile larva to its final substrate. Furthermore, they share developmental and functional features with vertebrate myoepithelial cells, vascular smooth muscle cells, and cardiomyocytes. We discuss these findings in the context of the proposed models of vertebrate smooth muscle and cardiomyocyte evolution.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures



Similar articles
-
Using CRISPR/Cas9 to identify genes required for mechanosensory neuron development and function.Biol Open. 2023 Sep 15;12(9):bio060002. doi: 10.1242/bio.060002. Epub 2023 Sep 5. Biol Open. 2023. PMID: 37589291 Free PMC article.
-
Specification of distinct cell types in a sensory-adhesive organ important for metamorphosis in tunicate larvae.PLoS Biol. 2024 Mar 13;22(3):e3002555. doi: 10.1371/journal.pbio.3002555. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38478577 Free PMC article.
-
Using CRISPR/Cas9 to identify genes required for mechanosensory neuron development and function.bioRxiv [Preprint]. 2023 May 8:2023.05.08.539861. doi: 10.1101/2023.05.08.539861. bioRxiv. 2023. Update in: Biol Open. 2023 Sep 15;12(9):bio060002. doi: 10.1242/bio.060002. PMID: 37214826 Free PMC article. Updated. Preprint.
-
The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning.Dev Biol. 2014 May 1;389(1):98-119. doi: 10.1016/j.ydbio.2014.01.019. Epub 2014 Feb 1. Dev Biol. 2014. PMID: 24491817 Review.
-
The origin and evolution of vertebrate neural crest cells.Open Biol. 2020 Jan;10(1):190285. doi: 10.1098/rsob.190285. Epub 2020 Jan 29. Open Biol. 2020. PMID: 31992146 Free PMC article. Review.
Cited by
-
Using CRISPR/Cas9 to identify genes required for mechanosensory neuron development and function.Biol Open. 2023 Sep 15;12(9):bio060002. doi: 10.1242/bio.060002. Epub 2023 Sep 5. Biol Open. 2023. PMID: 37589291 Free PMC article.
-
Specification of distinct cell types in a sensory-adhesive organ important for metamorphosis in tunicate larvae.PLoS Biol. 2024 Mar 13;22(3):e3002555. doi: 10.1371/journal.pbio.3002555. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38478577 Free PMC article.
-
Using CRISPR/Cas9 to identify genes required for mechanosensory neuron development and function.bioRxiv [Preprint]. 2023 May 8:2023.05.08.539861. doi: 10.1101/2023.05.08.539861. bioRxiv. 2023. Update in: Biol Open. 2023 Sep 15;12(9):bio060002. doi: 10.1242/bio.060002. PMID: 37214826 Free PMC article. Updated. Preprint.
-
MRTF specifies a muscle-like contractile module in Porifera.Nat Commun. 2022 Jul 15;13(1):4134. doi: 10.1038/s41467-022-31756-9. Nat Commun. 2022. PMID: 35840552 Free PMC article.
-
The evolutionary origins of the vertebrate olfactory system.Open Biol. 2020 Dec;10(12):200330. doi: 10.1098/rsob.200330. Epub 2020 Dec 23. Open Biol. 2020. PMID: 33352063 Free PMC article. Review.
References
-
- Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, Wagner GP. The origin and evolution of cell types. Nat Rev Genet. 2016;17:744–757. - PubMed
-
- Beh J, Shi W, Levine M, Davidson B, Christiaen L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development. 2007;134:3297–3305. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

