Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review
- PMID: 32774837
- PMCID: PMC7399607
- DOI: 10.2217/hep-2020-0024
Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review
Abstract
Aim: To describe the epidemiologic, humanistic and economic burdens of hepatocellular carcinoma (HCC) in the USA.
Materials & methods: Studies describing the epidemiology and economic burden from national cohorts, any economic models, or any humanistic burden studies published 2008-2018 were systematically searched.
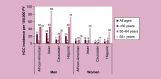
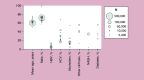
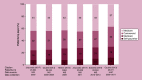
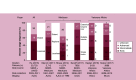
Results: HCC incidence was 9.5 per 100,000 person-years in most recent data, but was ∼100-times higher among patients with hepatitis/cirrhosis. Approximately a third of patients were diagnosed with advanced disease. Patients with HCC experienced poor quality of life. Direct costs were substantial and varied based on underlying demographics, disease stage and treatment received. Between 25-77% of patients did not receive surgical, locoregional or systemic treatment.
Conclusion: Better treatments are needed to extend survival and improve quality of life for patients with HCC.
Keywords: epidemiology; hepatocellular carcinoma; pharmacoeconomics; risk factors; staging.
© 2020 Aly et al.
Conflict of interest statement
Financial & competing interests disclosure Funding for this study was provided by AstraZeneca. A Aly, Y Doleh and F Benavente are employees and stockholders of AstraZeneca. S Ronnebaum and D Patel are employees of Pharmerit - an OPEN Health Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures




References
-
- Global Cancer Observatory (2018). https://gco.iarc.fr/today/home
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 69(1), 7–34 (2019). - PubMed
-
- Islami F, Goding Sauer A, Miller KD. et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 68(1), 31–54 (2018). - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
