Adaptation via pleiotropy and linkage: Association mapping reveals a complex genetic architecture within the stickleback Eda locus
- PMID: 32774879
- PMCID: PMC7403726
- DOI: 10.1002/evl3.175
Adaptation via pleiotropy and linkage: Association mapping reveals a complex genetic architecture within the stickleback Eda locus
Abstract
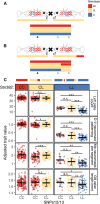
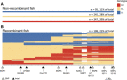
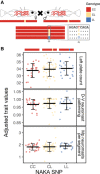
Genomic mapping of the loci associated with phenotypic evolution has revealed genomic "hotspots," or regions of the genome that control multiple phenotypic traits. This clustering of loci has important implications for the speed and maintenance of adaptation and could be due to pleiotropic effects of a single mutation or tight genetic linkage of multiple causative mutations affecting different traits. The threespine stickleback (Gasterosteus aculeatus) is a powerful model for the study of adaptive evolution because the marine ecotype has repeatedly adapted to freshwater environments across the northern hemisphere in the last 12,000 years. Freshwater ecotypes have repeatedly fixed a 16 kilobase haplotype on chromosome IV that contains Ectodysplasin (Eda), a gene known to affect multiple traits, including defensive armor plates, lateral line sensory hair cells, and schooling behavior. Many additional traits have previously been mapped to a larger region of chromosome IV that encompasses the Eda freshwater haplotype. To identify which of these traits specifically map to this adaptive haplotype, we made crosses of rare marine fish heterozygous for the freshwater haplotype in an otherwise marine genetic background. Further, we performed fine-scale association mapping in a fully interbreeding, polymorphic population of freshwater stickleback to disentangle the effects of pleiotropy and linkage on the phenotypes affected by this haplotype. Although we find evidence that linked mutations have small effects on a few phenotypes, a small 1.4-kb region within the first intron of Eda has large effects on three phenotypic traits: lateral plate count, and both the number and patterning of the posterior lateral line neuromasts. Thus, the Eda haplotype is a hotspot of adaptation in stickleback due to both a small, pleiotropic region affecting multiple traits as well as multiple linked mutations affecting additional traits.
Keywords: Adaptation; Ectodysplasin (Eda); Gasterosteus aculeatus; association mapping; lateral line neuromasts; lateral plates; linkage; pleiotropy; threespine stickleback.
© 2020 The Authors. Evolution Letters published by Wiley Periodicals LLC on behalf of Society for the Study of Evolution (SSE) and European Society for Evolutionary Biology (ESEB).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Albert, A. Y. K. , Sawaya S., Vines T. H., Knecht A. K., Miller C. T., Summers B. R., et al. 2008. The genetics of adaptive shape shift in stickleback: pleiotropy and effect size. Evolution 62:76–85. - PubMed
-
- Barrett, R. D. H. , and Hoekstra H. E.. 2011. Molecular spandrels: tests of adaptation at the genetic level. Nat. Rev. Genet. 12:767–780. - PubMed
-
- Barrett, R. D. H. , Rogers S. M., and Schluter D.. 2008. Natural selection on a major armor gene in threespine stickleback. Science 322:255–257. - PubMed

