Efficacy of Diltiazem for the Control of Blood Pressure in Puerperal Patients with Severe Preeclampsia: A Randomized, Single-Blind, Controlled Trial
- PMID: 32774912
- PMCID: PMC7397380
- DOI: 10.1155/2020/5347918
Efficacy of Diltiazem for the Control of Blood Pressure in Puerperal Patients with Severe Preeclampsia: A Randomized, Single-Blind, Controlled Trial
Abstract
Background: Postpartum preeclampsia is a serious disease related to high blood pressure that occurs commonly within the first six days after delivery.
Objective: To evaluate if diltiazem improves blood pressure parameters in early puerperium patients with severe preeclampsia. Methodology. A randomized, single-blind longitudinal clinical trial of 42 puerperal patients with severe preeclampsia was carried out. Patients were randomized into two groups: the experimental group (n = 21) received diltiazem (60 mg) and the control group (n = 21) received nifedipine (10 mg). Both drugs were orally administered every 8 hours. Systolic, diastolic, and mean blood pressures as well as the heart rate were recorded and analyzed (two-way repeated measures ANOVA) at baseline and after 6, 12, 18, 24, 30, 36, 42, and 48 hours. Primary outcome measures were all the aforementioned blood pressure parameters. Secondary outcome measures included the number of hypertension and hypotension episodes along with the length of stay in the intensive care unit.
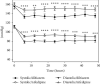
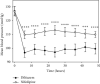
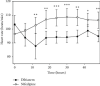
Results: No statistical differences were found between groups (diltiazem vs. nifedipine) regarding basal blood pressure parameters. Interarm differences in blood pressure (systolic, diastolic, and mean) and heart rate were statistically significant between treatment groups from 6 to 48 hours. Patients in the diltiazem group had lower blood pressure levels than patients in the nifedipine group. Significantly, patients who received diltiazem had fewer hypertension and hypotension episodes and stayed fewer days in the intensive care unit than those treated with nifedipine.
Conclusions: Diltiazem controlled arterial hypertension in a more effective and uniform manner in patients under study than nifedipine. Patients treated with diltiazem had fewer collateral effects and spent less time in the hospital. This trial is registered with NCT04222855.
Copyright © 2020 Gilberto Arias-Hernández et al.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial.Am J Obstet Gynecol. 2018 Jun;218(6):616.e1-616.e8. doi: 10.1016/j.ajog.2018.02.016. Epub 2018 Mar 2. Am J Obstet Gynecol. 2018. PMID: 29505772 Free PMC article. Clinical Trial.
-
Oral combined hydrochlorothiazide and lisinopril vs nifedipine for postpartum hypertension: a comparative-effectiveness pilot randomized controlled trial.Am J Obstet Gynecol. 2023 May;228(5):571.e1-571.e10. doi: 10.1016/j.ajog.2023.01.015. Epub 2023 Feb 12. Am J Obstet Gynecol. 2023. PMID: 36787814 Clinical Trial.
-
A comparative study of long acting diltiazem (Tildiem LA) with sustained release nifedipine (nifedipine SR) and bendrofluazide in the treatment of mild to moderate hypertension.Acta Cardiol. 1994;49(3):251-65. Acta Cardiol. 1994. PMID: 7941918 Clinical Trial.
-
Furosemide in postpartum management of severe preeclampsia: A randomized controlled trial.Hypertens Pregnancy. 2017 Feb;36(1):84-89. doi: 10.1080/10641955.2016.1239735. Epub 2016 Nov 11. Hypertens Pregnancy. 2017. PMID: 27835048 Clinical Trial.
-
Clinical experience with a once-daily, extended-release formulation of diltiazem in the treatment of hypertension.Am J Med. 1992 Aug 31;93(2A):56S-64S. doi: 10.1016/0002-9343(92)90295-m. Am J Med. 1992. PMID: 1519637 Review.
Cited by
-
Oral antihypertensive agents and diuretics in the management of hypertension in postpartum women: a systematic review and meta-analysis.BMJ Open. 2024 Dec 20;14(12):e086208. doi: 10.1136/bmjopen-2024-086208. BMJ Open. 2024. PMID: 39806714 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

