PARP6 suppresses the proliferation and metastasis of hepatocellular carcinoma by degrading XRCC6 to regulate the Wnt/β-catenin pathway
- PMID: 32775003
- PMCID: PMC7407359
PARP6 suppresses the proliferation and metastasis of hepatocellular carcinoma by degrading XRCC6 to regulate the Wnt/β-catenin pathway
Abstract
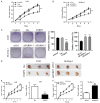
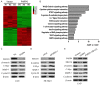
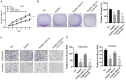
PARP6 belongs to the mono-ADP-ribosyltransferase family and has been shown to be involved in the genesis and development of some tumours. However, the role of PARP6 in hepatocellular carcinoma (HCC) development remains to be fully elucidated. In the current study, we demonstrated that PARP6 was expressed at a low level in HCC cells and was negatively related to the degree of tumour differentiation. Additionally, silencing PARP6 led to an increase in the proliferation, invasion and migration ability of HCC cells in both in vitro and in vivo assays. Conversely, an elevation in the PARP6 expression level had the opposite effect. Through gene chip analysis combined with experimental verification, we confirmed that PARP6 can inhibit the expression of XRCC6 by inducing degradation and thus affect the Wnt/β-Catenin signalling pathway, which contributes to the suppression of HCC. Further mechanistic investigation demonstrated that the ubiquitin ligase HDM2 can interact with PARP6 and XRCC6, and mediated the regulatory effect of PARP6 on XRCC6 degradation. Taking together, PARP6 appears to inhibit HCC progression through the XRCC6/Wnt/β-catenin signal axis and could be used as a biomarker for the clinical monitoring of HCC development.
Keywords: HCC; PARP6; XRCC6; cell proliferation; metastasis.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures




Similar articles
-
Suppression of Long Noncoding RNA SNHG1 Inhibits the Development of Hypopharyngeal Squamous Cell Carcinoma via Increasing PARP6 Expression.Evid Based Complement Alternat Med. 2022 Jul 5;2022:1562219. doi: 10.1155/2022/1562219. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35836822 Free PMC article.
-
CUL4B activates Wnt/β-catenin signalling in hepatocellular carcinoma by repressing Wnt antagonists.J Pathol. 2015 Apr;235(5):784-95. doi: 10.1002/path.4492. Epub 2015 Jan 23. J Pathol. 2015. PMID: 25430888
-
Metallothionein 1H (MT1H) functions as a tumor suppressor in hepatocellular carcinoma through regulating Wnt/β-catenin signaling pathway.BMC Cancer. 2017 Feb 28;17(1):161. doi: 10.1186/s12885-017-3139-2. BMC Cancer. 2017. PMID: 28241806 Free PMC article.
-
MicroRNA-194 inhibits cell invasion and migration in hepatocellular carcinoma through PRC1-mediated inhibition of Wnt/β-catenin signaling pathway.Dig Liver Dis. 2019 Sep;51(9):1314-1322. doi: 10.1016/j.dld.2019.02.012. Epub 2019 Mar 1. Dig Liver Dis. 2019. PMID: 30948333
-
LRP16 prevents hepatocellular carcinoma progression through regulation of Wnt/β-catenin signaling.J Mol Med (Berl). 2018 Jun;96(6):547-558. doi: 10.1007/s00109-018-1639-4. Epub 2018 May 11. J Mol Med (Berl). 2018. PMID: 29748698
Cited by
-
ADP-Ribosylation as Post-Translational Modification of Proteins: Use of Inhibitors in Cancer Control.Int J Mol Sci. 2021 Oct 7;22(19):10829. doi: 10.3390/ijms221910829. Int J Mol Sci. 2021. PMID: 34639169 Free PMC article. Review.
-
X-ray cross-complementing family: the bridge linking DNA damage repair and cancer.J Transl Med. 2023 Sep 7;21(1):602. doi: 10.1186/s12967-023-04447-2. J Transl Med. 2023. PMID: 37679817 Free PMC article. Review.
-
Characteristics of hypoxic tumor microenvironment in non-small cell lung cancer, involving molecular patterns and prognostic signature.Transl Lung Cancer Res. 2021 May;10(5):2132-2147. doi: 10.21037/tlcr-20-1314. Transl Lung Cancer Res. 2021. PMID: 34164265 Free PMC article.
-
Research Advances in the Role of the Poly ADP Ribose Polymerase Family in Cancer.Front Oncol. 2021 Dec 16;11:790967. doi: 10.3389/fonc.2021.790967. eCollection 2021. Front Oncol. 2021. PMID: 34976832 Free PMC article. Review.
-
PARP (Poly ADP-ribose Polymerase) Family in Health and Disease.MedComm (2020). 2025 Sep 1;6(9):e70314. doi: 10.1002/mco2.70314. eCollection 2025 Sep. MedComm (2020). 2025. PMID: 40904702 Free PMC article. Review.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118–123. - PubMed
-
- Posavec Marjanovic M, Crawford K, Ahel I. PARP, transcription and chromatin modeling. Semin Cell Dev Biol. 2017;63:102–113. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
