Enhancing Chimeric Antigen Receptor T Cell Anti-tumor Function through Advanced Media Design
- PMID: 32775494
- PMCID: PMC7397397
- DOI: 10.1016/j.omtm.2020.07.008
Enhancing Chimeric Antigen Receptor T Cell Anti-tumor Function through Advanced Media Design
Abstract
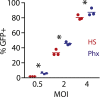
Effective chimeric antigen receptor (CAR)-T cell therapy is dependent on optimal cell culture methods conducive to the activation and expansion of T cells ex vivo, as well as infection with CAR. Media formulations used in CAR-T cell manufacturing have not been optimized for gene delivery, cell expansion, and overall potency. Bioactive components and derivatives that support the generation of functionally-competent T cell progeny with long-lasting persistence are largely undefined. Current media formulations rely on fetal bovine serum (FBS) or human serum (HS), which suffer from a lack of consistency or supply issues. We recognize that components of blood cellular fractions that are absent in serum may have therapeutic value. Here we investigate whether a concentrated growth factor extract, purified from human transfusion grade whole blood fractions, and marketed as PhysiologixTM xeno-free (XF) hGFC (Phx), supports CAR-T cell expansion and function. We show that Phx supports T cell proliferation in clinical and research-grade media. We also show that Phx treatment enhances lentiviral-mediated gene expression across a wide range of multiplicity of infections (MOIs). We compared the ability of anti-GD-2 CAR-T cells expanded ex vivo in medium conditioned with either Phx or HS to clear tumor burden in a human xenograft model of neuroblastoma. We show that T cells expanded in Phx have superior engraftment and potency in vivo, as well as CAR-induced cytolytic activity in vitro. Metabolomic profiling revealed several factors unique to Phx that may have relevance for CAR-T cell preclinical discovery, process development, and manufacturing. In particular, we show that carnosine, a biogenic amine modestly enriched in Phx relative to HS, enhances lentiviral gene delivery in activated T cells. By limiting extracellular acidification, carnosine enhances the metabolic fitness of T cells, shifting their metabolic profile from an acidic, stressed state toward an oxidative, energetic state. These findings are very informative regarding potential derivatives to include in medium customized for gene delivery and overall potency for T cell adoptive immunotherapies.
© 2020 The Author(s).
Figures





References
-
- June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. - PubMed
-
- O’Sullivan D., Sanin D.E., Pearce E.J., Pearce E.L. Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 2019;19:324–335. - PubMed
-
- Bieback K., Fernandez-Muñoz B., Pati S., Schäfer R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: a joint publication from the AABB and the International Society for Cell & Gene Therapy. Cytotherapy. 2019;21:911–924. - PubMed
-
- Carson B.P., Patel B., Amigo-Benavent M., Pauk M., Kumar Gujulla S., Murphy S.M., Kiely P.A., Jakeman P.M. Regulation of muscle protein synthesis in an in vitro cell model using ex vivo human serum. Exp. Physiol. 2018;103:783–789. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

