A polymer-based systemic hemostatic agent
- PMID: 32775633
- PMCID: PMC7394519
- DOI: 10.1126/sciadv.aba0588
A polymer-based systemic hemostatic agent
Abstract
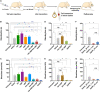
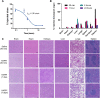
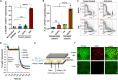
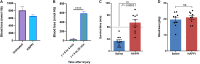
Uncontrolled noncompressible hemorrhage is a major cause of mortality following traumatic injuries in civilian and military populations. An injectable hemostat for point-of-care treatment of noncompressible hemorrhage represents an urgent medical need. Here, we describe an injectable hemostatic agent via polymer peptide interfusion (HAPPI), a hyaluronic acid conjugate with a collagen-binding peptide and a von Willebrand factor-binding peptide. HAPPI exhibited selective binding to activated platelets and promoted their accumulation at the wound site in vitro. In vivo studies in mouse tail vein laceration model demonstrated a reduction of >97% in both bleeding time and blood loss. A 284% improvement in the survival time was observed in the rat inferior vena cava traumatic model. Lyophilized HAPPI could be stably stored at room temperature for several months and reconstituted during therapeutic intervention. HAPPI provides a potentially clinically translatable intravenous hemostat.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- D. Berwick, A. Downey, Elizabeth Cornett, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury (National Academies Press, 2016). - PubMed
-
- S. Shackelford, B. J. Eastridge, in Damage Control Resuscitation (Springer, 2020), pp. 31–40.
-
- Rhee P., Joseph B., Pandit V., Aziz H., Vercruysse G., Kulvatunyou N., Friese R. S., Increasing trauma deaths in the United States. Ann. Surg. 260, 13–21 (2014). - PubMed
-
- Davis J. S., Satahoo S. S., Butler F. K., Dermer H., Naranjo D., Julien K., Van Haren R. M., Namias N., Blackbourne L. H., Schulman C. I., An analysis of prehospital deaths: Who can we save? J. Trauma Acute Care Surg. 77, 213–218 (2014). - PubMed
-
- Spinella P. C., Zero preventable deaths after traumatic injury: An achievable goal. J. Trauma Acute Care Surg. 82, S2–S8 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

