Gamma radiation improves AD pathogenesis in APP/PS1 mouse model by potentiating insulin sensitivity
- PMID: 32775714
- PMCID: PMC7399127
- DOI: 10.1016/j.heliyon.2020.e04499
Gamma radiation improves AD pathogenesis in APP/PS1 mouse model by potentiating insulin sensitivity
Abstract
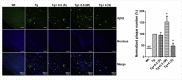
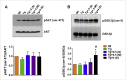
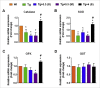
Alzheimer's disease (AD) is the largest unmet medical complication. The devastation caused by the disease can be assumed from the disease symptoms like speech impairment, loss of self-awareness, acute memory loss etc. The individuals suffering from AD completely depend on caregivers and have to bear the high cost of treatment which increases the socio-economic burden on the society. Recent studies have shown that radiation exposure can have therapeutic effects when given in suitable amount for a specific time period. Therefore, we investigated the role of gamma irradiation in AD pathogenesis. The effect of radiation on amelioration of disease progression was studied in AD transgenic mice model (APP/PS1). Our in-vivo studies using APP/PS1 mice demonstrated that a single dose of 4.0 Gy gamma irradiation improves AD associated behavioral impairment. Radiation exposure also increased the level of anti-oxidant enzymes and reduced the astrocyte activation in the brain of APP/PS1 mice. A significant reduction was observed in AD associated proteins (APP, pTau, BACE) and neurofibrillary tangle formations (NFTs). Exposure to a single dose of 4 Gy gamma radiation also increased glucose metabolic functionality in AD transgenic mouse model. The kinases involved in insulin signaling such as GSK, ERK and JNK were also found to be modulated. However, an increased level of GSK3β (ser 9) was observed, which could be responsible for downregulating ERK and JNK phosphorylation. This resulted in a decrease in neurofibrillary tangle formations and amyloid deposition. The reduced hyperphosphorylation of Tau can be attributed to the increased level of GSK3β (ser 9) downregulating ERK and JNK phosphorylation. Thus, a single dose of 4 Gy gamma irradiation was found to have therapeutic benefits in treating AD via potentiating insulin signaling in APP/PS1 transgenic mice.
Keywords: APP/PS1 mice; Alzheimer's disease; Biochemistry; Brain insulin resistance; Cognition; Gamma radiation; Molecular biology; Neurology; Neuroscience; Radiology.
© 2020 Published by Elsevier Ltd.
Figures






References
-
- Heron M. Deaths: leading causes for 2016. Natl. Vital Stat. Rep. 2018;67:1–77. - PubMed
-
- Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., Arvanitakis Z., Schneider J.A., Wolf B.A., Bennett D.A., Trojanowski J.Q., Arnold S.E. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 2012;122:1316–1338. - PMC - PubMed
-
- Hossain S., Akaike T., Chowdhury E.H. Current approaches for drug delivery to central nervous system. Curr. Drug Deliv. 2010;7:389–397. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

