The GluN3 subunit regulates ion selectivity within native N-methyl-d-aspartate receptors
- PMID: 32775760
- PMCID: PMC7399132
- DOI: 10.1016/j.ibror.2020.07.009
The GluN3 subunit regulates ion selectivity within native N-methyl-d-aspartate receptors
Abstract
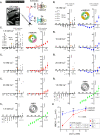
Glutamatergic N-methyl-d-aspartate receptors (NMDARs) are heterotetrameric proteins whose subunits are derived from three gene families, GRIN1 (codes for GluN1), GRIN2 (GluN2) and GRIN3 (GluN3). In addition to providing binding sites for glutamate and the co-agonist glycine, these subunits in their di (d-) and tri (t-) heteromeric configurations regulate various aspects of receptor function in the brain. For example, the decay kinetics of NMDAR-mediated synaptic currents depend on the type of GluN2 subunit (GluN2A-GluN2D) in the receptor subunit composition. While much is known about the contributions of GluN1 and GluN2 to d-NMDAR function, we know comparatively little about how GluN3 influences the function of t-NMDARs composed of one or more subunits from each of the three gene families. We report here that in addition to altering kinetics and voltage-dependent properties, the GluN3 subunit endows these receptors with ion selectivity wherein influx of Ca2+ is preferred over Na+. This became apparent in the process of assessing Ca2+ permeability through these receptors and is of significance given that NMDARs are generally believed to be nonselective to cations and increased selectivity can lead to enhanced permeability. This was true of two independent brain regions where t-NMDARs are expressed, the somatosensory cortex, where both receptor subtypes are expressed at separate inputs onto single neurons, and the entorhinal cortex, where they are co-expressed at individual synaptic inputs. Based on this data and the sequence of amino acids lining selectivity filters within these subunits, we propose GluN3 to be a regulatory subunit for ion selectivity in t-NMDARs.
Keywords: Electrophysiology; Entorhinal cortex; GluN3; Ion selectivity; Ion-substitution experiments; Somatosensory cortex; Triheteromeric NMDA receptors.
© 2020 The Author(s).
Figures



References
-
- Burnashev N., Monyer H., Seeburg P.H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
-
- Burnashev N., Schoepfer R., Monyer H., Ruppersberg J.P., Gunther W., Seeburg P.H., Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

