Recent Advances in the Synthesis of Perimidines and their Applications
- PMID: 32776212
- PMCID: PMC7415412
- DOI: 10.1007/s41061-020-00307-5
Recent Advances in the Synthesis of Perimidines and their Applications
Abstract
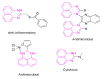
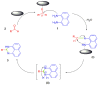
Perimidines are versatile scaffolds and a fascinating class of N-heterocycles that have evolved significantly in recent years due to their immense applications in life sciences, medical sciences, and industrial chemistry. Their ability of molecular interaction with different proteins, complex formation with metals, and distinct behavior in various ranges of light makes them more appealing and challenging for future scientists. Various novel technologies have been developed for the selective synthesis of perimidines and their conjugated derivatives. These methods extend to the preparation of different bioactive and industrially applicable molecules. This review aims to present the most recent advancements in perimidine synthesis under varied conditions like MW radiation, ultrasound, and grinding using different catalysts such as ionic liquids, acid, metal, and nanocatalyst and also under green environments like catalyst and solvent-free synthesis. The applications of perimidine derivatives in drug discovery, polymer chemistry, photo sensors, dye industries, and catalytic activity in organic synthesis are discussed in this survey. This article is expected to be a systematic, authoritative, and critical review on the chemistry of perimidines that compiles most of the state-of-art innovation in this area.
Keywords: Biologic activity; Catalysis; N-heterocycles; Perimidines; Synthesis.
Conflict of interest statement
The authors confirm that this article has no conflicts of interest.
Figures










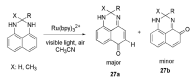
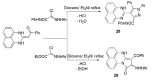
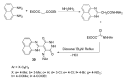
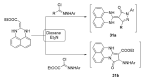


References
-
- O’Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989;37(3):233–309. - PubMed
-
- Elce M, Dearden CE, Matutes E, Garcia-Talavera J, Rohatiner AZS, Johnson SAN, O'Connor NTJ, Haynes A, Osuji N, Forconi F, Lauria F, Catovsky D. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145(6):733–740. - PubMed
-
- Arora P, Arora V, Lamba HS, Wadhwa D. Importance of heterocyclic chemistry: a review. Int J Pharm Sci. 2012;3(9):2947–2954.
-
- Rudi A, Shmul G, Benayahu Y, Kashman Y. Sinularectin, a new diterpenoid from the soft coral Sinularia erecta. Tetrahedron Lett. 2006;47(17):2937–2939.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
