Graphene impregnated electrospun nanofiber sensing materials: a comprehensive overview on bridging laboratory set-up to industry
- PMID: 32776254
- PMCID: PMC7417471
- DOI: 10.1186/s40580-020-00237-4
Graphene impregnated electrospun nanofiber sensing materials: a comprehensive overview on bridging laboratory set-up to industry
Abstract
Owing to the unique structural characteristics as well as outstanding physio-chemical and electrical properties, graphene enables significant enhancement with the performance of electrospun nanofibers, leading to the generation of promising applications in electrospun-mediated sensor technologies. Electrospinning is a simple, cost-effective, and versatile technique relying on electrostatic repulsion between the surface charges to continuously synthesize various scalable assemblies from a wide array of raw materials with diameters down to few nanometers. Recently, electrospun nanocomposites have emerged as promising substrates with a great potential for constructing nanoscale biosensors due to their exceptional functional characteristics such as complex pore structures, high surface area, high catalytic and electron transfer, controllable surface conformation and modification, superior electric conductivity and unique mat structure. This review comprehends graphene-based nanomaterials (GNMs) (graphene, graphene oxide (GO), reduced GO and graphene quantum dots) impregnated electrospun polymer composites for the electro-device developments, which bridges the laboratory set-up to the industry. Different techniques in the base polymers (pre-processing methods) and surface modification methods (post-processing methods) to impregnate GNMs within electrospun polymer nanofibers are critically discussed. The performance and the usage as the electrochemical biosensors for the detection of wide range analytes are further elaborated. This overview catches a great interest and inspires various new opportunities across a wide range of disciplines and designs of miniaturized point-of-care devices.
Keywords: Electrochemical biosensors; Electrospinning; Electrospun nanofibers; Graphene; Graphene oxide; Graphene quantum dots; Nanocomposites; Reduced graphene oxide.
Conflict of interest statement
The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper.
Figures



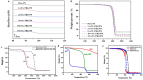
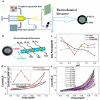
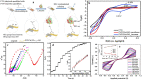
References
-
- Asif MH, Razaq A, Akbar N, Danielsson B, Sultana I. Facile synthesis of multisegment Au/Ni/Au nanowire for high performance electrochemical glucose sensor. Mater. Res. Express. 2019;6:95028.
-
- Asmatulu R, Veisi Z, Uddin MN, Mahapatro A. Highly sensitive and reliable electrospun polyaniline nanofiber based biosensor as a robust platform for COX-2 enzyme detections. Fibers Polym. 2019;20:966–974. doi: 10.1007/s12221-019-1096-x. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
