Multiparameter flow cytometric detection and quantification of senescent cells in vitro
- PMID: 32776262
- PMCID: PMC7541365
- DOI: 10.1007/s10522-020-09893-9
Multiparameter flow cytometric detection and quantification of senescent cells in vitro
Abstract
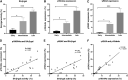
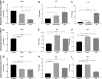
It has been over half a century since cellular senescence was first noted and characterized, and yet no consensus senescent marker has been reliably established. This challenge is compounded by the complexity and heterogenic phenotypes of senescent cells. This necessitates the use of multiple biomarkers to confidently characterise senescent cells. Despite cytochemical staining of senescence associated-beta-galactosidase being a single marker approach, as well as being time and labour-intensive, it remains the most popular detection method. We have developed an alternative flow cytometry-based method that simultaneously quantifies multiple senescence markers at a single-cell resolution. In this study, we applied this assay to the quantification of both replicative and induced senescent primary cells. Using this assay, we were able to quantify the activity level of SA β-galactosidase, the expression level of p16INK4a and γH2AX in these cell populations. Our results show this flow cytometric approach to be sensitive, robust, and consistent in discriminating senescent cells in different cell senescence models. A strong positive correlation between these commonly- used senescence markers was demonstrated. The method described in this paper can easily be scaled up to accommodate high-throughput screening of senescent cells in applications such as therapeutic cell preparation, and in therapy-induced senescence following cancer treatment.
Keywords: Aging; Flow cytometry; Mesenchymal stem cells; Quantification; Senescence.
Conflict of interest statement
There are no conflicts of interest.
Figures




References
-
- Althubiti M, Macip S. Detection of senescent cells by extracellular markers using a flow cytometry-based approach. Methods Mol Biol. 2017;1534:147. - PubMed
-
- Angello JC, Pendergrass WR, Norwood TH, Prothero J. Proliferative potential of human fibroblasts: an inverse dependence on cell size. J Cell Physiol. 1987;132(1):125–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

