Effects of RAGE inhibition on the progression of the disease in hSOD1G93A ALS mice
- PMID: 32776498
- PMCID: PMC7415959
- DOI: 10.1002/prp2.636
Effects of RAGE inhibition on the progression of the disease in hSOD1G93A ALS mice
Abstract
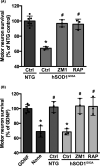
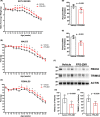
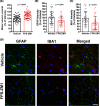
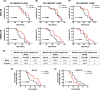
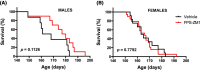
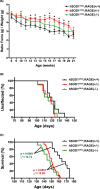
Astrocytes play a key role in the progression of amyotrophic lateral sclerosis (ALS) by actively inducing the degeneration of motor neurons. Motor neurons isolated from receptor for advanced glycation end products (RAGE)-knockout mice are resistant to the neurotoxic signal derived from ALS-astrocytes. Here, we confirmed that in a co-culture model, the neuronal death induced by astrocytes over-expressing the ALS-linked mutant hSOD1G93A is prevented by the addition of the RAGE inhibitors FPS-ZM1 or RAP. These inhibitors also prevented the motor neuron death induced by spinal cord extracts from symptomatic hSOD1G93A mice. To evaluate the relevance of this neurotoxic mechanism in ALS pathology, we assessed the therapeutic potential of FPS-ZM1 in hSOD1G93A mice. FPS-ZM1 treatment significantly improved hind-limb grip strength in hSOD1G93A mice during the progression of the disease, reduced the expression of atrophy markers in the gastrocnemius muscle, improved the survival of large motor neurons, and reduced gliosis in the ventral horn of the spinal cord. However, we did not observe a statistically significant effect of the drug in symptoms onset nor in the survival of hSOD1G93A mice. Maintenance of hind-limb grip strength was also observed in hSOD1G93A mice with RAGE haploinsufficiency [hSOD1G93A ;RAGE(+/-)], further supporting the beneficial effect of RAGE inhibition on muscle function. However, no benefits were observed after complete RAGE ablation. Moreover, genetic RAGE ablation significantly shortened the median survival of hSOD1G93A mice. These results indicate that the advance of new therapies targeting RAGE in ALS demands a better understanding of its physiological role in a cell type/tissue-specific context.
Keywords: Atrogin-1; MuRF1; advanced glycation end products receptor; amyotrophic lateral sclerosis; astrocytes; motor neurons.
© 2020 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Microglia RAGE exacerbates the progression of neurodegeneration within the SOD1G93A murine model of amyotrophic lateral sclerosis in a sex-dependent manner.J Neuroinflammation. 2021 Jun 15;18(1):139. doi: 10.1186/s12974-021-02191-2. J Neuroinflammation. 2021. PMID: 34130712 Free PMC article.
-
Absence of Receptor for Advanced Glycation End Product (RAGE) Reduces Inflammation and Extends Survival in the hSOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis.Mol Neurobiol. 2020 Oct;57(10):4143-4155. doi: 10.1007/s12035-020-02019-9. Epub 2020 Jul 16. Mol Neurobiol. 2020. PMID: 32676989
-
Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article.
-
Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis.Mol Neurobiol. 2012 Feb;45(1):30-42. doi: 10.1007/s12035-011-8217-x. Epub 2011 Nov 10. Mol Neurobiol. 2012. PMID: 22072396 Free PMC article. Review.
-
Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis.Curr Pharm Des. 2017;23(33):5010-5021. doi: 10.2174/1381612823666170622095802. Curr Pharm Des. 2017. PMID: 28641533 Free PMC article. Review.
Cited by
-
Targeting RAGE to prevent SARS-CoV-2-mediated multiple organ failure: Hypotheses and perspectives.Life Sci. 2021 May 1;272:119251. doi: 10.1016/j.lfs.2021.119251. Epub 2021 Feb 23. Life Sci. 2021. PMID: 33636175 Free PMC article. Review.
-
Nicotinamide Adenine Dinucleotide Precursor Supplementation Modulates Neurite Complexity and Survival in Motor Neurons from Amyotrophic Lateral Sclerosis Models.Antioxid Redox Signal. 2024 Sep;41(7-9):573-589. doi: 10.1089/ars.2023.0360. Epub 2024 Jul 8. Antioxid Redox Signal. 2024. PMID: 38504592
-
Role of RAGE in the Pathogenesis of Neurological Disorders.Neurosci Bull. 2022 Oct;38(10):1248-1262. doi: 10.1007/s12264-022-00878-x. Epub 2022 Jun 21. Neurosci Bull. 2022. PMID: 35729453 Free PMC article. Review.
-
scAAV9-VEGF alleviates symptoms of amyotrophic lateral sclerosis (ALS) mice through regulating aromatase.Exp Brain Res. 2023 Dec;241(11-12):2817-2827. doi: 10.1007/s00221-023-06721-7. Epub 2023 Oct 26. Exp Brain Res. 2023. PMID: 37882882
-
Roles of the Receptor for Advanced Glycation End Products and Its Ligands in the Pathogenesis of Alzheimer's Disease.Int J Mol Sci. 2025 Jan 5;26(1):403. doi: 10.3390/ijms26010403. Int J Mol Sci. 2025. PMID: 39796257 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

