Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS-CoV-2 infection: A retrospective cohort study
- PMID: 32776534
- PMCID: PMC7436522
- DOI: 10.1002/jmv.26407
Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS-CoV-2 infection: A retrospective cohort study
Abstract
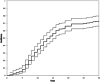
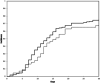
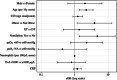
As it has been shown that lopinavir (LPV) and hydroxychloroquine (HCQ) have in vitro activity against coronaviruses, they were used to treat COVID-19 during the first wave of the epidemic in Lombardy, Italy. To compare the rate of clinical improvement between those who started LPV/ritonavir (LPV/r)+HCQ within 5 days of symptom onset (early treatment, ET) and those who started later (delayed treatment, DT). This was a retrospective intent-to-treat analysis of the hospitalized patients who started LPV/r + HCQ between 21 February and 20 March 2020. The association between the timing of treatment and the probability of 30-day mortality was assessed using univariable and multivariable logistic models. The study involved 172 patients: 43 (25%) in the ET and 129 (75%) in the DT group. The rate of clinical improvement increased over time to 73.3% on day 30, without any significant difference between the two groups (Gray's test P = .213). After adjusting for potentially relevant clinical variables, there was no significant association between the timing of the start of treatment and the probability of 30-day mortality (adjusted odds ratio [aOR] ET vs DT = 1.45, 95% confidence interval 0.50-4.19). Eight percent of the patients discontinued the treatment becausebecause of severe gastrointestinal disorders attributable to LPV/r. The timing of the start of LPV/r + HCQ treatment does not seem to affect the clinical course of hospitalized patients with COVID-19. Together with the severe adverse events attributable to LPV/r, this raises concerns about the benefit of using this combination to treat COVID-19.
Keywords: COVID-19; antiviral treatment; early; hydroxychloroquine; lopinavir; mortality.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
AG has received consultancy fees from Mylan and nonfinancial educational support from Gilead. GR has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen and Roche. SR has received grants, fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead and Janssen. CG has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen. DC has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, Gilead, Janssen. SA has received support for research activities from Pfizer and Merck Sharp & Dome. MG has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen and Roche. GP, ALR, LO, FC, LP, LB, GC, SP, CB, VM, CC, EC and RC have nothing to declare.
Figures
References
-
- World Health Organization . Coronavirus disease (COVID‐19) Situation Report– 111. Data as received by WHO from national authorities by 10:00 CEST, 15 June 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Covid‐19 Regione Lombardia, 16 June 2020. https://experience.arcgis.com/experience/0a5dfcc103d0468bbb6b14e713ec1e30/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

