Molecular characteristics and physiological roles of Na+ -K+ -Cl- cotransporter 2
- PMID: 32776569
- PMCID: PMC7818487
- DOI: 10.1002/jcp.29997
Molecular characteristics and physiological roles of Na+ -K+ -Cl- cotransporter 2
Abstract
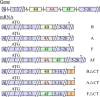
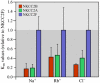
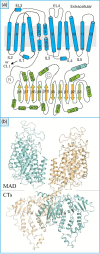
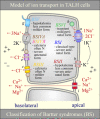
Na+ -K+ -Cl- cotransporter 2 (NKCC2; SLC12A1) is an integral membrane protein that comes as three splice variants and mediates the cotranslocation of Na+ , K+ , and Cl- ions through the apical membrane of the thick ascending loop of Henle (TALH). In doing so, and through the involvement of other ion transport systems, it allows this nephron segment to reclaim a large fraction of the ultrafiltered Na+ , Cl- , Ca2+ , Mg2+ , and HCO3- loads. The functional relevance of NKCC2 in human is illustrated by the many abnormalities that result from the inactivation of this transport system through the use of loop diuretics or in the setting of inherited disorders. The following presentation aims at discussing the physiological roles and molecular characteristics of Na+ -K+ -Cl- cotransport in the TALH and those of the individual NKCC2 splice variants more specifically. Many of the historical and recent data that have emerged from the experiments conducted will be outlined and their larger meaning will also be placed into perspective with the aid of various hypotheses.
Keywords: Bartter syndrome; Na+-K+-Cl− cotransporter 2; cation-Cl− cotransporter; high blood pressure; loop of Henle; renal physiology.
© 2020 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Acuna, R. , Martinez‐de‐la‐Maza, L. , Ponce‐Coria, J. , Vazquez, N. , Ortal‐Vite, P. , Pacheco‐Alvarez, D. , … Gamba, G. (2011). Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. Journal of Hypertension, 29(3), 475–483. 10.1097/HJH.0b013e328341d0fd - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

