Testosterone supplementation upregulates androgen receptor expression and translational capacity during severe energy deficit
- PMID: 32776828
- PMCID: PMC7750513
- DOI: 10.1152/ajpendo.00157.2020
Testosterone supplementation upregulates androgen receptor expression and translational capacity during severe energy deficit
Abstract
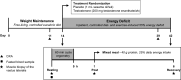
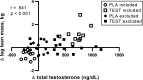
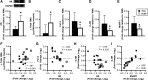
Testosterone supplementation during energy deficit promotes whole body lean mass accretion, but the mechanisms underlying that effect remain unclear. To elucidate those mechanisms, skeletal muscle molecular adaptations were assessed from muscle biopsies collected before, 1 h, and 6 h after exercise and a mixed meal (40 g protein, 1 h postexercise) following 14 days of weight maintenance (WM) and 28 days of an exercise- and diet-induced 55% energy deficit (ED) in 50 physically active nonobese men treated with 200 mg testosterone enanthate/wk (TEST) or placebo (PLA) during the ED. Participants (n = 10/group) exhibiting substantial increases in leg lean mass and total testosterone (TEST) were compared with those exhibiting decreases in both of these measures (PLA). Resting androgen receptor (AR) protein content was higher and fibroblast growth factor-inducible 14 (Fn14), IL-6 receptor (IL-6R), and muscle ring-finger protein-1 gene expression was lower in TEST vs. PLA during ED relative to WM (P < 0.05). Changes in inflammatory, myogenic, and proteolytic gene expression did not differ between groups after exercise and recovery feeding. Mechanistic target of rapamycin signaling (i.e., translational efficiency) was also similar between groups at rest and after exercise and the mixed meal. Muscle total RNA content (i.e., translational capacity) increased more during ED in TEST than PLA (P < 0.05). These findings indicate that attenuated proteolysis at rest, possibly downstream of AR, Fn14, and IL-6R signaling, and increased translational capacity, not efficiency, may drive lean mass accretion with testosterone administration during energy deficit.
Keywords: androgen receptor; inflammation; muscle mass; myonuclear accretion; negative energy balance; translational capacity.
Conflict of interest statement
J.C.R. and K.M.G. reported that their institution received funding from the US Department of Defense for work associated with this publication. H.R.L. reported receiving personal fees from Pfizer, Inc., for work outside this publication. All remaining authors declare no conflicts of interest, financial or otherwise.
Figures


References
-
- Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res 63: 7106–7112, 2003. - PubMed
-
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi:10.3945/ajcn.2010.29819. - DOI - PubMed
-
- Berryman CE, Sepowitz JJ, McClung HL, Lieberman HR, Farina EK, McClung JP, Ferrando AA, Pasiakos SM. Supplementing an energy adequate, higher protein diet with protein does not enhance fat-free mass restoration after short-term severe negative energy balance. J Appl Physiol (1985) 122: 1485–1493, 2017. doi:10.1152/japplphysiol.01039.2016. - DOI - PubMed
-
- Berryman CE, Young AJ, Karl JP, Kenefick RW, Margolis LM, Cole RE, Carbone JW, Lieberman HR, Kim IY, Ferrando AA, Pasiakos SM. Severe negative energy balance during 21 d at high altitude decreases fat-free mass regardless of dietary protein intake: a randomized controlled trial. FASEB J 32: 894–905, 2018. doi:10.1096/fj.201700915R. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

