Incidence and etiology of clinically-attended, antibiotic-treated diarrhea among children under five years of age in low- and middle-income countries: Evidence from the Global Enteric Multicenter Study
- PMID: 32776938
- PMCID: PMC7444547
- DOI: 10.1371/journal.pntd.0008520
Incidence and etiology of clinically-attended, antibiotic-treated diarrhea among children under five years of age in low- and middle-income countries: Evidence from the Global Enteric Multicenter Study
Abstract
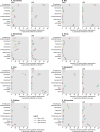
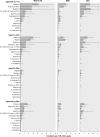
Diarrhea is a leading cause of antibiotic consumption among children in low- and middle-income countries. While vaccines may prevent diarrhea infections for which children often receive antibiotics, the contribution of individual enteropathogens to antibiotic use is minimally understood. We used data from the Global Enteric Multicenter Study (GEMS) to estimate pathogen-specific incidence of antibiotic-treated diarrhea among children under five years old residing in six countries of sub-Saharan Africa and South Asia before rotavirus vaccine implementation. GEMS was an age-stratified, individually-matched case-control study. Stool specimens were obtained from children presenting to sentinel health clinics with newly-onset, acute diarrhea (including moderate-to-severe and less-severe diarrhea) as well as matched community controls without diarrhea. We used data from conventional and quantitative molecular diagnostic assays applied to stool specimens to estimate the proportion of antibiotic-treated diarrhea cases attributable to each pathogen. Antibiotics were administered or prescribed to 9,606 of 12,109 moderate-to-severe cases and 1,844 of 3,174 less-severe cases. Across all sites, incidence rates of clinically-attended, antibiotic-treated diarrhea were 12.2 (95% confidence interval: 9.0-17.8), 10.2 (7.4-13.9) and 1.9 (1.3-3.0) episodes per 100 child-years at risk at ages 6 weeks to 11 months, 12-23 months, and 24-59 months, respectively. Based on the recommendation for antibiotic treatment to be reserved for cases with dysentery, we estimated a ratio of 12.6 (8.6-20.8) inappropriately-treated diarrhea cases for each appropriately-treated case. Rotavirus, adenovirus serotypes 40/41, Shigella, sapovirus, Shiga toxin-producing Escherichia coli, and Cryptosporidium were the leading antibiotic-treated diarrhea etiologies. Rotavirus caused 29.2% (24.5-35.2%) of antibiotic-treated cases, including the largest share in both the first and second years of life. Shigella caused 14.9% (11.4-18.9%) of antibiotic-treated cases, and was the leading etiology at ages 24-59 months. Our findings should inform the prioritization of vaccines with the greatest potential to reduce antibiotic exposure among children.
Conflict of interest statement
JAL has received grants and consulting fees from Pfizer Inc. and Merck, Sharp, & Dohme unrelated to the submitted work. KLK has received grants from Merck, Sharp & Dohme unrelated to the submitted work. All other authors declare no competing interests exist.
Figures


References
-
- World Health Organization. Global action plan on antimicrobial resistance. WHO Press; 2015;: 1–28. - PubMed
-
- Fink G, D’Acremont V, Leslie HH, Cohen J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis 2020. 10.1016/S1473-3099(19)30572-9 - DOI - PubMed

