Eight years of sales surveillance of antimicrobials for veterinary use in Germany-What are the perceptions?
- PMID: 32776971
- PMCID: PMC7416935
- DOI: 10.1371/journal.pone.0237459
Eight years of sales surveillance of antimicrobials for veterinary use in Germany-What are the perceptions?
Abstract
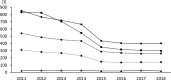
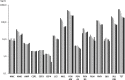
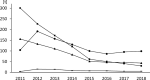
A surveillance system for sales volumes of antimicrobial agents for veterinary use was established in Germany in 2011. Since then, pharmaceutical companies and wholesalers have been legally obliged to report annual volumes of veterinary antimicrobial products sold to veterinary practices or clinics located in Germany. The evaluation of sales volumes for eight consecutive years resulted in a considerable total decrease by 58% from 1706 tons to 722 tons. During the investigation period, two legally binding measures to control the risk of antimicrobial resistance resulting from the veterinary use of antimicrobials were introduced, a) the German treatment frequencies benchmarking in 2014 and b) the obligation to conduct susceptibility testing for the use of cephalosporins of the 3rd and 4th generation and of fluoroquinolones in 2018. Both had a marked impact on sales volumes. Nonetheless, the category of Critically Important Antimicrobials as defined by the World Health Organization kept accounting for the highest share on sales volumes in Germany in 2018 with 403 tons, despite an overall reduction by 53%. Sales surveillance is considered essential for data retrieval on a global scale and inter-country comparison. However, the usability of a surveillance system based on sales data for risk management of antimicrobial resistance has limitations. The German system does not include off-label use of antimicrobial products authorized for human medicine and does not allow for identification of areas of high risk according to animal species, farm and production types and indications for treatment. For further reduction and enhanced promotion of a prudent use of antimicrobials, targeted measures would be required that could only be deducted from use data collected at farm or veterinary practice level. A surveillance system based on use data is currently lacking in Germany but will be established according to Regulation (EU) 2019/6 on veterinary medicinal products.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18: 268–281. doi: 10.1111/j.1469-0691.2011.03570.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

