Sperm associated antigen 9 promotes oncogenic KSHV-encoded interferon regulatory factor-induced cellular transformation and angiogenesis by activating the JNK/VEGFA pathway
- PMID: 32776977
- PMCID: PMC7446834
- DOI: 10.1371/journal.ppat.1008730
Sperm associated antigen 9 promotes oncogenic KSHV-encoded interferon regulatory factor-induced cellular transformation and angiogenesis by activating the JNK/VEGFA pathway
Erratum in
-
Correction: Sperm associated antigen 9 promotes oncogenic KSHV-encoded interferon regulatory factor-induced cellular transformation and angiogenesis by activating the JNK/VEGFA pathway.PLoS Pathog. 2022 Jan 7;18(1):e1010232. doi: 10.1371/journal.ppat.1010232. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 34995339 Free PMC article.
Abstract
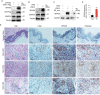
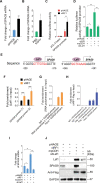
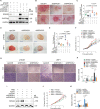
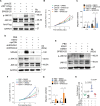
Kaposi's sarcoma (KS), caused by Kaposi's sarcoma-associated herpesvirus (KSHV), is a highly angioproliferative disseminated tumor of endothelial cells commonly found in AIDS patients. We have recently shown that KSHV-encoded viral interferon regulatory factor 1 (vIRF1) mediates KSHV-induced cell motility (PLoS Pathog. 2019 Jan 30;15(1):e1007578). However, the role of vIRF1 in KSHV-induced cellular transformation and angiogenesis remains unknown. Here, we show that vIRF1 promotes angiogenesis by upregulating sperm associated antigen 9 (SPAG9) using two in vivo angiogenesis models including the chick chorioallantoic membrane assay (CAM) and the matrigel plug angiogenesis assay in mice. Mechanistically, vIRF1 interacts with transcription factor Lef1 to promote SPAG9 transcription. vIRF1-induced SPAG9 promotes the interaction of mitogen-activated protein kinase kinase 4 (MKK4) with JNK1/2 to increase their phosphorylation, resulting in enhanced VEGFA expression, angiogenesis, cell proliferation and migration. Finally, genetic deletion of ORF-K9 from KSHV genome abolishes KSHV-induced cellular transformation and impairs angiogenesis. Our results reveal that vIRF1 transcriptionally activates SPAG9 expression to promote angiogenesis and tumorigenesis via activating JNK/VEGFA signaling. These novel findings define the mechanism of KSHV induction of the SPAG9/JNK/VEGFA pathway and establish the scientific basis for targeting this pathway for treating KSHV-associated cancers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

