Pulmonary function analysis in cotton rats after respiratory syncytial virus infection
- PMID: 32776985
- PMCID: PMC7416943
- DOI: 10.1371/journal.pone.0237404
Pulmonary function analysis in cotton rats after respiratory syncytial virus infection
Abstract
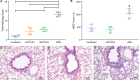
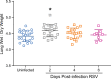
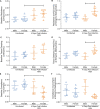
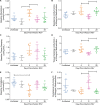
The cotton rat (Sigmodon hispidus) is an excellent small animal model for human respiratory viral infections such as human respiratory syncytial virus (RSV) and human metapneumovirus (HMPV). These respiratory viral infections, as well as other pulmonary inflammatory diseases such as asthma, are associated with lung mechanic disturbances. So far, the pathophysiological effects of viral infection and allergy on cotton rat lungs have not been measured, although this information might be an important tool to determine the efficacy of vaccine and drug candidates. To characterize pulmonary function in the cotton rat, we established forced oscillation technique in uninfected, RSV infected and HDM sensitized cotton rats, and characterized pulmonary inflammation, mucus production, pulmonary edema, and oxygenation. There was a gender difference after RSV infection, with females demonstrating airway hyper-responsiveness while males did not. Female cotton rats 2dpi had a mild increase in pulmonary edema (wet: dry weight ratios). At day 4 post infection, female cotton rats demonstrated mild pulmonary inflammation, no increase in mucus production or reduction in oxygenation. Pulmonary function was not significantly impaired after RSV infection. In contrast, cotton rats sensitized to HDM demonstrated airway hyper-responsiveness with a significant increase in pulmonary inflammation, increase in baseline tissue damping, and a decrease in baseline pulmonary compliance. In summary, we established baseline data for forced oscillation technique and other respiratory measures in the cotton rat and used it to analyze respiratory diseases in cotton rats.
Conflict of interest statement
Genentech provided the corresponding author with a research fellowship that aided in the following research to be conducted. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Characterization of Cotton Rat (Sigmodon hispidus) Eosinophils, Including Their Response to Respiratory Syncytial Virus Infection.Comp Med. 2018 Feb 1;68(1):31-40. Comp Med. 2018. PMID: 29460719 Free PMC article.
-
Virus-like particle vaccines containing F or F and G proteins confer protection against respiratory syncytial virus without pulmonary inflammation in cotton rats.Hum Vaccin Immunother. 2017 May 4;13(5):1031-1039. doi: 10.1080/21645515.2016.1272743. Epub 2017 Jan 27. Hum Vaccin Immunother. 2017. PMID: 28129031 Free PMC article.
-
Acute and Chronic Airway Disease After Human Respiratory Syncytial Virus Infection in Cotton Rats (Sigmodon hispidus).Comp Med. 2015 Aug;65(4):315-26. Comp Med. 2015. PMID: 26310461 Free PMC article.
-
Cotton rat model for testing vaccines and antivirals against respiratory syncytial virus.Antivir Chem Chemother. 2018 Jan-Dec;26:2040206618770518. doi: 10.1177/2040206618770518. Antivir Chem Chemother. 2018. PMID: 29768937 Free PMC article. Review.
-
Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S66-74; discussion S74-5. doi: 10.1097/01.inf.0000053888.67311.1d. Pediatr Infect Dis J. 2003. PMID: 12671455 Review.
Cited by
-
Characteristics of mucin hypersecretion in different inflammatory patterns based on endotypes of chronic rhinosinusitis.Clin Transl Allergy. 2024 Jan;14(1):e12334. doi: 10.1002/clt2.12334. Clin Transl Allergy. 2024. PMID: 38282195 Free PMC article. Review.
-
Facile, Rapid, and Low-Cost Detection for Influenza Viruses and Respiratory Syncytial Virus Based on a Catalytic DNA Assembly Circuit.ACS Omega. 2022 Apr 19;7(17):15074-15081. doi: 10.1021/acsomega.2c00882. eCollection 2022 May 3. ACS Omega. 2022. PMID: 35557683 Free PMC article.
-
Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection.Viruses. 2023 Nov 19;15(11):2276. doi: 10.3390/v15112276. Viruses. 2023. PMID: 38005952 Free PMC article.
-
Immunopathology of RSV: An Updated Review.Viruses. 2021 Dec 10;13(12):2478. doi: 10.3390/v13122478. Viruses. 2021. PMID: 34960746 Free PMC article. Review.
-
Immunogenicity and inflammatory properties of respiratory syncytial virus attachment G protein in cotton rats.PLoS One. 2021 Feb 18;16(2):e0246770. doi: 10.1371/journal.pone.0246770. eCollection 2021. PLoS One. 2021. PMID: 33600439 Free PMC article.
References
-
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(1):108–19. Epub 2006/12/05. 10.1038/modpathol.3800725 . - DOI - PubMed
-
- Zomer-Kooijker K, Uiterwaal CS, van der Gugten AC, Wilbrink B, Bont LJ, van der Ent CK. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. The European respiratory journal. 2014;44(3):666–74. Epub 2014/07/06. 10.1183/09031936.00009314 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

