An engineered factor Va prevents bleeding induced by direct-acting oral anticoagulants by different mechanisms
- PMID: 32777068
- PMCID: PMC7422119
- DOI: 10.1182/bloodadvances.2020001699
An engineered factor Va prevents bleeding induced by direct-acting oral anticoagulants by different mechanisms
Abstract
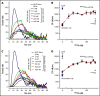
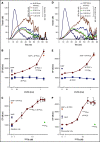
Control of bleeding with direct-acting oral anticoagulants (DOACs) remains an unmet clinical need. Activated superFactor V (superFVa) is an engineered activated protein C (APC)-resistant FVa variant with enhanced procoagulant activity resulting from an A2/A3 domain disulfide bond and was studied here for control of DOAC-induced bleeding. SuperFVa reversed bleeding induced by FXa inhibitors (rivaroxaban, apixaban), and the FIIa inhibitor dabigatran in BalbC mice. The blocking anti-protein C and APC [(A)PC] antibody SPC-54 also reduced FXa inhibitor induced bleeding similar to superFVa, whereas dabigatran-induced bleeding was not affected. This indicated that sufficient APC was generated to contribute to bleeding in the presence of FXa inhibitors, but not in the presence of dabigatran, suggesting that mechanisms contributing to bleeding differed for FXa and FIIa inhibitors. Despite different mechanisms contributing to bleeding, superFVa effectively reduced bleeding for all DOACs, indicating the versatility of superFVa's properties that contribute to its universal prohemostatic effects for DOAC associated bleeding. Supported by thrombin generation assays on endothelial cells in normal plasma spiked with DOACs and patient plasma anticoagulated with DOACs, 3 complementary mechanisms were identified by which superFVa achieved DOAC class-independent prohemostatic efficiency. These mechanisms are resistance to inactivation by APC, overcoming the FV activation threshold, and maximizing the efficiency of the prothrombinase complex when the available FXa is increased by FVIIa-based prohemostatics. In summary, it is this versatility of superFVa that delineates it from other prohemostatic agents as a promising class-independent rescue agent in bleeding situations associated with DOACs.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.v.D. has received honoraria for participating in scientific advisory board panels, consulting, and speaking engagements for Takeda, Biomarin, Bioverativ/Sanofi, Novo Nordisk, and Uniqure, and reports research support from Pfizer and Sanofi. The University of California San Diego and The Scripps Research Institute hold intellectual property rights related to superFVa on which A.v.D., A.J.G., J.H.G., and L.O.M. are listed as inventors. A.v.D., A.J.G., and L.O.M. are founders of Hematherix LLC, a biotech company that is developing superFVa therapy for bleeding complications. A.v.D. and L.O.M. are members of the board of directors of Hematherix LLC. The remaining authors declare no competing financial interests.
Figures





References
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. - PubMed
-
- Lee CH, Fang CC, Tsai LM, et al. Changing treatment patterns in patients with venous thromboembolism in Taiwan. Circ J. 2020;84(2):283-293. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. - PubMed
-
- Connolly SJ, Eikelboom J, Joyner C, et al. ; AVERROES Steering Committee and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817. - PubMed

