Sustained fetal hematopoiesis causes juvenile death from leukemia: evidence from a dual-age-specific mouse model
- PMID: 32777070
- PMCID: PMC7422126
- DOI: 10.1182/bloodadvances.2020002326
Sustained fetal hematopoiesis causes juvenile death from leukemia: evidence from a dual-age-specific mouse model
Abstract
It is not clear whether disrupted age-specific hematopoiesis contributes to the complex manifestations in leukemia patients who carry identical mutations, particularly in pediatric and adult patients with similar clinical characteristics. By studying a dual-age-specific mouse model, we demonstrate that (1) loss of Pten during the fetal-to-adult hematopoiesis switch (hematopoiesis switch) causes sustained fetal hematopoiesis, resulting in death in juvenile leukemia; (2) myeloid-biased hematopoiesis in juvenile mice is associated with the sustained fetal properties of hematopoietic stem cells (HSCs); (3) the age specificity of juvenile myelomonocytic leukemia depends on the copy number of Pten and Nf1; (4) single-allelic Pten deletion during the hematopoiesis switch causes constitutive activation of MAPK in juvenile mice with Nf1 loss of heterozygosity (LOH); and (5) Nf1 LOH causes monocytosis in juvenile mice with Pten haploinsufficiency but does not cause lethality until adulthood. Our data suggest that 1 copy of Pten is sufficient to maintain an intact negative-feedback loop of the Akt pathway and HSC function in reconstitution, despite MAPK being constitutively activated in juvenile Pten+/ΔNf1LOH mice. However, 2 copies of Pten are required to maintain the integrity of the MAPK pathway in juvenile mice with Nf1 haploinsufficiency. Our data indicate that previous investigations of Pten function in wild-type mice may not reflect the impact of Pten loss in mice with Nf1 mutations or other genetic defects. We provide a proof of concept that disassociated age-specific hematopoiesis contributes to leukemogenesis and pediatric demise.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Figures

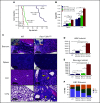



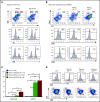
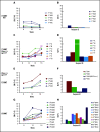


References
-
- Niemeyer CM, Arico M, Basso G, et al. ; European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) . Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. Blood. 1997;89(10):3534-3543. - PubMed
-
- Sakaguchi H, Okuno Y, Muramatsu H, et al. . Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat Genet. 2013;45(8):937-941. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

