Viral structural proteins as targets for antivirals
- PMID: 32777753
- PMCID: PMC8809080
- DOI: 10.1016/j.coviro.2020.07.001
Viral structural proteins as targets for antivirals
Abstract
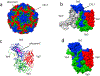
Viral structural proteins are emerging as effective targets for new antivirals. In a viral lifecycle, the capsid must assemble, disassemble, and respond to host proteins, all at the right time and place. These reactions work within a narrow range of conditions, making them susceptible to small molecule interference. In at least three specific viruses, this approach has had met with preliminary success. In rhinovirus and poliovirus, compounds like pleconaril bind capsid and block RNA release. Bevirimat binds to Gag protein in HIV, inhibiting maturation. In Hepatitis B virus, core protein allosteric modulators (CpAMs) promote spontaneous assembly of capsid protein leading to empty and aberrant particles. Despite the biological diversity between viruses and the chemical diversity between antiviral molecules, we observe common features in these antivirals' mechanisms of action. These approaches work by stabilizing protein-protein interactions.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
AZ acknowledges an interest in biotech companies involved in developing antivirals directed at HBV; these exercised no influence in the preparation of this manuscript.
Figures

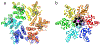
References
-
-
Tanner EJ, Liu HM, Oberste MS, Pallansch M, Collett MS, Kirkegaard K. 2014. Dominant drug targets suppress the emergence of antiviral resistance. Elife 3.
This paper describes how viral capsids and other viral oligomers can remain susceptible to antiviral agents even when selective pressure leads to the appearance of resistant mutants. The presence of even a relatively small fraction of sensitive wild type protein can result in drug-sensitivity of the whole oligomer.
-
-
- Zlotnick A. 1994. To build a virus capsid. An equilibrium model of the self assembly of polyhedral protein complexes. J Mol Biol 241:59–67. - PubMed
-
- Johnston IG, Louis AA, Doye JP. 2010. Modelling the self-assembly of virus capsids. J Phys Condens Matter 22:104101. - PubMed
-
-
Perlmutter JD, Hagan MF. 2015. Mechanisms of virus assembly. Annu Rev Phys Chem 66:217–39.
This encyclopedic review describes the state of the art for modelling virus capsid assembly, touching on ordinary differential equation, Gillespie (stochastic event), and dynamic models. There are also substantive descriptions of means to analyze experimental data in light of model predictions.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

