Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis
- PMID: 32777817
- PMCID: PMC7116780
- DOI: 10.1182/blood.2020006157
Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis
Abstract
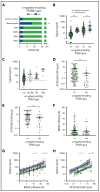
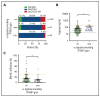
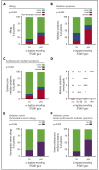
Mastocytosis is a hematopoietic neoplasm characterized by expansion of KIT D816V-mutated clonal mast cells in various organs and severe or even life-threatening anaphylactic reactions. Recently, hereditary α-tryptasemia (HαT) has been described as a common genetic trait with increased copy numbers of the α-tryptase encoding gene, TPSAB1, and associated with an increased basal serum tryptase level and a risk of mast cell activation. The purpose of our study was to elucidate the clinical relevance of HαT in patients with mastocytosis. TPSAB1 germline copy number variants were assessed by digital polymerase chain reaction in 180 mastocytosis patients, 180 sex-matched control subjects, 720 patients with other myeloid neoplasms, and 61 additional mastocytosis patients of an independent validation cohort. α-Tryptase encoding TPSAB1 copy number gains, compatible with HαT, were identified in 17.2% of mastocytosis patients and 4.4% of the control population (P < .001). Patients with HαT exhibited higher tryptase levels than patients without HαT (median tryptase in HαT+ cases: 49.6 ng/mL vs HαT- cases: 34.5 ng/mL, P = .004) independent of the mast cell burden. Hymenoptera venom hypersensitivity reactions and severe cardiovascular mediator-related symptoms/anaphylaxis were by far more frequently observed in mastocytosis patients with HαT than in those without HαT. Results were confirmed in an independent validation cohort. The high prevalence of HαT in mastocytosis hints at a potential pathogenic role of germline α-tryptase encoding TPSAB1 copy number gains in disease evolution. Together, our data suggest that HαT is a novel emerging robust biomarker in mastocytosis that is useful for determining the individual patient´s risk of developing severe anaphylaxis.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.H. received honoraria and research grants from Novartis, Roche, Beckman Coulter, Pfizer, Celgene, and Bristol-Myers Squibb. P.V. served as a consultant in a global Novartis trial investigating the effects of midostaurin in patients with advanced systemic mastocytosis and received honoraria and research grants from Novartis, Blueprint, Pfizer, Ariad, Incyte, Celgene, and Deciphera. W.R.S. received honoraria from Novartis. G.U. received honoraria and grants from Ariad, Astellas, AstraZeneca, Incyte, Novartis, and Pfizer. K.G.V. received honoraria from Pfizer, Novartis, Incyte, and Sanofi-Aventis. H.G. received honoraria from Novartis, Celgene, Janssen, and AOP Orphan. The remaining authors declare no competing financial interests.
Figures
Comment in
-
What your HαT says about you.Blood. 2021 Jan 14;137(2):151-153. doi: 10.1182/blood.2020008466. Blood. 2021. PMID: 33443557 No abstract available.
References
-
- Horny H-P, Metcalfe DD, Akin C, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon, France: International Agency for Research on Cancer (IARC); 2017. pp. 62–69.
-
- Akin C, Valent P. Diagnostic criteria and classification of mastocytosis in 2014. Immunol Allergy Clin North Am. 2014;34(2):207–218. - PubMed
-
- Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163–172. - PubMed
-
- Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

