A cluster randomized controlled trial for the Evaluation of routinely Measured PATient reported outcomes in HemodialYsis care (EMPATHY): a study protocol
- PMID: 32778102
- PMCID: PMC7418420
- DOI: 10.1186/s12913-020-05557-z
A cluster randomized controlled trial for the Evaluation of routinely Measured PATient reported outcomes in HemodialYsis care (EMPATHY): a study protocol
Abstract
Background: Kidney failure requiring dialysis is associated with poor health outcomes and health-related quality of life (HRQL). Patient-reported outcome measures (PROMs) capture symptom burden, level of functioning and other outcomes from a patient perspective, and can support clinicians to monitor disease progression, address symptoms, and facilitate patient-centered care. While evidence suggests the use of PROMs in clinical practice can lead to improved patient experience in some settings, the impact on patients' health outcomes and experiences is not fully understood, and their cost-effectiveness in clinical settings is unknown. This study aims to fill these gaps by evaluating the effectiveness and cost-effectiveness of routinely measuring PROMs on patient-reported experience, clinical outcomes, HRQL, and healthcare utilization.
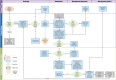
Methods: The EMPATHY trial is a pragmatic multi-centre cluster randomized controlled trial that will implement and evaluate the use of disease-specific and generic PROMs in three kidney care programs in Canada. In-centre hemodialysis units will be randomized into four groups, whereby patients: 1) complete a disease-specific PROM; 2) complete a generic PROM; 3) complete both types of PROMs; 4) receive usual care and do not complete any PROMs. While clinical care pathways are available to all hemodialysis units in the study, for the three active intervention groups, the results of the PROMs will be linked to treatment aids for clinicians and patients. The primary outcome of this study is patient-provider communication, assessed by the Communication Assessment Tool (CAT). Secondary outcomes include patient management and symptoms, use of healthcare services, and the costs of implementing this intervention will also be estimated. The present protocol fulfilled the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) checklist.
Discussion: While using PROMs in clinical practice is supported by theory and rationale, and may engage patients and enhance their role in decisions regarding their care and outcomes, the best approach of their use is still uncertain. It is important to rigorously evaluate such interventions and investments to ensure they provide value for patients and health systems.
Trial registration: Protocol version (1.0) and trial registration data are available on www.clinicaltrials.gov , identifier: NCT03535922 , registered May 24, 2018.
Keywords: Controlled trial; Hemodialysis; Kidney failure; Patient-reported outcome measures; Quality improvement; Symptom burden.
Conflict of interest statement
Fatima Al Sayah and Jeffrey Johnson are members of the EuroQol Group. J Johnson is a member of the Board of Directors for the EuroQol Research Foundation. Braden Manns is co-Principal Investigator of the Can-SOLVE CKD Network. All authors have no other conflicts of interest to declare.
Figures

Similar articles
-
"You need a team": perspectives on interdisciplinary symptom management using patient-reported outcome measures in hemodialysis care-a qualitative study.J Patient Rep Outcomes. 2023 Jan 20;7(1):3. doi: 10.1186/s41687-022-00538-8. J Patient Rep Outcomes. 2023. PMID: 36662325 Free PMC article.
-
Patient-reported outcome measures in the care of in-centre hemodialysis patients.J Patient Rep Outcomes. 2021 Oct 12;5(Suppl 2):93. doi: 10.1186/s41687-021-00365-3. J Patient Rep Outcomes. 2021. PMID: 34637030 Free PMC article.
-
Evaluating the Population-Based Usage and Benefit of Digitally Collected Patient-Reported Outcomes and Experiences in Patients With Chronic Diseases: The PROMchronic Study Protocol.JMIR Res Protoc. 2024 Aug 5;13:e56487. doi: 10.2196/56487. JMIR Res Protoc. 2024. PMID: 39102279 Free PMC article.
-
Gaps in patient-reported outcome measures in randomized clinical trials of cardiac catheter ablation: a systematic review.Eur Heart J Qual Care Clin Outcomes. 2020 Oct 1;6(4):234-242. doi: 10.1093/ehjqcco/qcaa022. Eur Heart J Qual Care Clin Outcomes. 2020. PMID: 32170937
-
A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs).Qual Life Res. 2019 Mar;28(3):567-592. doi: 10.1007/s11136-018-2016-z. Epub 2018 Oct 3. Qual Life Res. 2019. PMID: 30284183
Cited by
-
Screening for symptoms of anxiety and depression in patients treated with renal replacement therapy: utility of the Edmonton Symptom Assessment System-Revised.Qual Life Res. 2022 Feb;31(2):597-605. doi: 10.1007/s11136-021-02910-5. Epub 2021 Jun 17. Qual Life Res. 2022. PMID: 34138450
-
"You need a team": perspectives on interdisciplinary symptom management using patient-reported outcome measures in hemodialysis care-a qualitative study.J Patient Rep Outcomes. 2023 Jan 20;7(1):3. doi: 10.1186/s41687-022-00538-8. J Patient Rep Outcomes. 2023. PMID: 36662325 Free PMC article.
-
Protocol for 'Re: CBT Dialysis': a realist evaluation-why, for whom and in what circumstances does cognitive behaviour therapy work for people with depressive symptoms receiving dialysis?BMJ Open. 2025 Jun 23;15(6):e090228. doi: 10.1136/bmjopen-2024-090228. BMJ Open. 2025. PMID: 40550722 Free PMC article.
-
How Are Albertans "Adjusting to and Coping With" Dialysis? A Cross-Sectional Survey.Can J Kidney Health Dis. 2022 Aug 23;9:20543581221118436. doi: 10.1177/20543581221118436. eCollection 2022. Can J Kidney Health Dis. 2022. PMID: 36046483 Free PMC article.
-
The role of kidney registries in expediting large-scale collection of patient-reported outcome measures for people with chronic kidney disease.Clin Kidney J. 2021 Mar 16;14(6):1495-1503. doi: 10.1093/ckj/sfab061. eCollection 2021 Jun. Clin Kidney J. 2021. PMID: 34276974 Free PMC article.
References
-
- Canadian Institute for Health Information. Organ replacement in Canada: CORR annual statistics, 2019. 2019 June 1, 2020]; Available from: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistic....
-
- Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. - PubMed
-
- Jablonski A. The multidimensional characteristics of symptoms reported by patients on hemodialysis. Nephrol Nurs J. 2007;34(1):29–37. - PubMed
-
- Lowney AC, et al. Understanding what influences the health-related quality of life of hemodialysis patients: a collaborative study in England and Ireland. J Pain Symptom Manag. 2015;50(6):778–785. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

