A seventeenth-century Mycobacterium tuberculosis genome supports a Neolithic emergence of the Mycobacterium tuberculosis complex
- PMID: 32778135
- PMCID: PMC7418204
- DOI: 10.1186/s13059-020-02112-1
A seventeenth-century Mycobacterium tuberculosis genome supports a Neolithic emergence of the Mycobacterium tuberculosis complex
Abstract
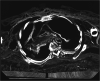
Background: Although tuberculosis accounts for the highest mortality from a bacterial infection on a global scale, questions persist regarding its origin. One hypothesis based on modern Mycobacterium tuberculosis complex (MTBC) genomes suggests their most recent common ancestor followed human migrations out of Africa approximately 70,000 years before present. However, studies using ancient genomes as calibration points have yielded much younger dates of less than 6000 years. Here, we aim to address this discrepancy through the analysis of the highest-coverage and highest-quality ancient MTBC genome available to date, reconstructed from a calcified lung nodule of Bishop Peder Winstrup of Lund (b. 1605-d. 1679).
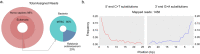
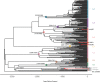
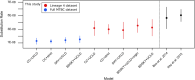
Results: A metagenomic approach for taxonomic classification of whole DNA content permitted the identification of abundant DNA belonging to the human host and the MTBC, with few non-TB bacterial taxa comprising the background. Genomic enrichment enabled the reconstruction of a 141-fold coverage M. tuberculosis genome. In utilizing this high-quality, high-coverage seventeenth-century genome as a calibration point for dating the MTBC, we employed multiple Bayesian tree models, including birth-death models, which allowed us to model pathogen population dynamics and data sampling strategies more realistically than those based on the coalescent.
Conclusions: The results of our metagenomic analysis demonstrate the unique preservation environment calcified nodules provide for DNA. Importantly, we estimate a most recent common ancestor date for the MTBC of between 2190 and 4501 before present and for Lineage 4 of between 929 and 2084 before present using multiple models, confirming a Neolithic emergence for the MTBC.
Keywords: Ancient DNA; Metagenomics; Molecular dating; Mycobacterium tuberculosis; Tuberculosis.
Conflict of interest statement
Not applicable.
Figures

References
-
- WHO. WHO | Tuberculosis (TB). WHO. 2018 [cited 2018 Nov 18]. Available from: http://www.who.int/tb/en/.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

