Ferroptosis, necroptosis, and pyroptosis in anticancer immunity
- PMID: 32778143
- PMCID: PMC7418434
- DOI: 10.1186/s13045-020-00946-7
Ferroptosis, necroptosis, and pyroptosis in anticancer immunity
Abstract
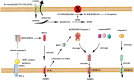
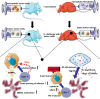
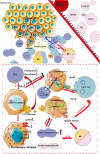
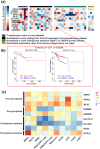
In recent years, cancer immunotherapy based on immune checkpoint inhibitors (ICIs) has achieved considerable success in the clinic. However, ICIs are significantly limited by the fact that only one third of patients with most types of cancer respond to these agents. The induction of cell death mechanisms other than apoptosis has gradually emerged as a new cancer treatment strategy because most tumors harbor innate resistance to apoptosis. However, to date, the possibility of combining these two modalities has not been discussed systematically. Recently, a few studies revealed crosstalk between distinct cell death mechanisms and antitumor immunity. The induction of pyroptosis, ferroptosis, and necroptosis combined with ICIs showed synergistically enhanced antitumor activity, even in ICI-resistant tumors. Immunotherapy-activated CD8+ T cells are traditionally believed to induce tumor cell death via the following two main pathways: (i) perforin-granzyme and (ii) Fas-FasL. However, recent studies identified a new mechanism by which CD8+ T cells suppress tumor growth by inducing ferroptosis and pyroptosis, which provoked a review of the relationship between tumor cell death mechanisms and immune system activation. Hence, in this review, we summarize knowledge of the reciprocal interaction between antitumor immunity and distinct cell death mechanisms, particularly necroptosis, ferroptosis, and pyroptosis, which are the three potentially novel mechanisms of immunogenic cell death. Because most evidence is derived from studies using animal and cell models, we also reviewed related bioinformatics data available for human tissues in public databases, which partially confirmed the presence of interactions between tumor cell death and the activation of antitumor immunity.
Keywords: Anticancer immunity; Ferroptosis; Necroptosis; Pyroptosis.
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. - PubMed
-
- Liao SK, Carr DH. Comparative immunogenicity of irradiated, neuraminidase treated, and fused cells of a strain-restricted sarcoma. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;82:133–142. - PubMed
-
- Bogden AE, Esber HJ. Influence of surgery, irradiation, chemotherapy, and immunotherapy on growth of a metastasizing rat mammary adenocarcinoma. Natl Cancer Inst Monogr. 1978;49:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

