Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism
- PMID: 32778145
- PMCID: PMC7418402
- DOI: 10.1186/s13041-020-00649-7
Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism
Abstract
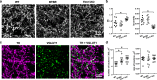
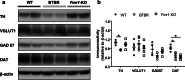
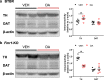
The dopamine (DA) system has a profound impact on reward-motivated behavior and is critically involved in neurodevelopmental disorders, such as autism spectrum disorder (ASD). Although DA defects are found in autistic patients, it is not well defined how the DA pathways are altered in ASD and whether DA can be utilized as a potential therapeutic agent for ASD. To this end, we employed a phenotypic and a genetic ASD model, i.e., Black and Tan BRachyury T+Itpr3tf/J (BTBR) mice and Fragile X Mental Retardation 1 knockout (Fmr1-KO) mice, respectively. Immunostaining of tyrosine hydroxylase (TH) to mark dopaminergic neurons revealed an overall reduction in the TH expression in the substantia nigra, ventral tegmental area and dorsal striatum of BTBR mice, as compared to C57BL/6 J wild-type ones. In contrast, Fmr1-KO animals did not show such an alteration but displayed abnormal morphology of TH-positive axons in the striatum with higher "complexity" and lower "texture". Both strains exhibited decreased expression of striatal dopamine transporter (DAT) and increased spatial coupling between vesicular glutamate transporter 1 (VGLUT1, a label for glutamatergic terminals) and TH signals, while GABAergic neurons quantified by glutamic acid decarboxylase 67 (GAD67) remained intact. Intranasal administration of DA rescued the deficits in non-selective attention, object-based attention and social approaching of BTBR mice, likely by enhancing the level of TH in the striatum. Application of intranasal DA to Fmr1-KO animals alleviated their impairment of social novelty, in association with reduced striatal TH protein. These results suggest that although the DA system is modified differently in the two ASD models, intranasal treatment with DA effectively rectifies their behavioral phenotypes, which may present a promising therapy for diverse types of ASD.
Keywords: Autism; BTBR; Fmr1; Fragile X syndrome; Social behavior; Striatum.
Conflict of interest statement
The authors declare no competing financial and non-financial interests in relation to this work. CM is an employee of M et P Pharma AG. However, the company did not play a role in any aspect of the study.
Figures



References
-
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. - PubMed
-
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. - PubMed
-
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):932–940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

