Plasticity and Spontaneous Activity Pulses in Disused Human Brain Circuits
- PMID: 32778224
- PMCID: PMC7419711
- DOI: 10.1016/j.neuron.2020.05.007
Plasticity and Spontaneous Activity Pulses in Disused Human Brain Circuits
Abstract
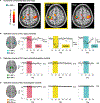
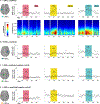
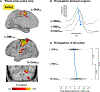
To induce brain plasticity in humans, we casted the dominant upper extremity for 2 weeks and tracked changes in functional connectivity using daily 30-min scans of resting-state functional MRI (rs-fMRI). Casting caused cortical and cerebellar regions controlling the disused extremity to functionally disconnect from the rest of the somatomotor system, while internal connectivity within the disused sub-circuit was maintained. Functional disconnection was evident within 48 h, progressed throughout the cast period, and reversed after cast removal. During the cast period, large, spontaneous pulses of activity propagated through the disused somatomotor sub-circuit. The adult brain seems to rely on regular use to maintain its functional architecture. Disuse-driven spontaneous activity pulses may help preserve functionally disconnected sub-circuits.
Keywords: ALFF; amplitude of low-frequency fluctuations; cerebellum; disuse; fMRI; functional connectivity; plasticity; primary motor cortex; resting state; spontaneous activity; supplementary motor area.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration Of Interests The authors declare the following competing financial interest: N.U.F.D. is co-founder of NOUS Imaging.
Figures



Comment in
-
Precision Neuroimaging Opens a New Chapter of Neuroplasticity Experimentation.Neuron. 2020 Aug 5;107(3):401-403. doi: 10.1016/j.neuron.2020.07.017. Neuron. 2020. PMID: 32758445 Free PMC article.
References
-
- Biswal B, Yetkin FZ, Haughton VM, and Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537–541. - PubMed
-
- Bullmore E, and Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10, 186–198. - PubMed
Publication types
MeSH terms
Grants and funding
- IK2 CX001680/CX/CSRD VA/United States
- K23 NS088590/NS/NINDS NIH HHS/United States
- T32 MH100019/MH/NIMH NIH HHS/United States
- F30 MH100872/MH/NIMH NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- R25 MH112473/MH/NIMH NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P01 NS080675/NS/NINDS NIH HHS/United States
- F31 NS110332/NS/NINDS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- K01 MH104592/MH/NIMH NIH HHS/United States
- K99 MH121518/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

