Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2
- PMID: 32778225
- PMCID: PMC7275151
- DOI: 10.1016/j.cell.2020.06.008
Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2
Abstract
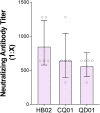
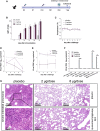
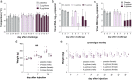
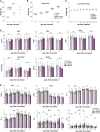
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) threatens global public health. The development of a vaccine is urgently needed for the prevention and control of COVID-19. Here, we report the pilot-scale production of an inactivated SARS-CoV-2 vaccine candidate (BBIBP-CorV) that induces high levels of neutralizing antibodies titers in mice, rats, guinea pigs, rabbits, and nonhuman primates (cynomolgus monkeys and rhesus macaques) to provide protection against SARS-CoV-2. Two-dose immunizations using 2 μg/dose of BBIBP-CorV provided highly efficient protection against SARS-CoV-2 intratracheal challenge in rhesus macaques, without detectable antibody-dependent enhancement of infection. In addition, BBIBP-CorV exhibits efficient productivity and good genetic stability for vaccine manufacture. These results support the further evaluation of BBIBP-CorV in a clinical trial.
Keywords: BBIBP-CorV; SARS-CoV-2; inactivated vaccine.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

