Synergy between amyloid-β and tau in Alzheimer's disease
- PMID: 32778792
- PMCID: PMC11831977
- DOI: 10.1038/s41593-020-0687-6
Synergy between amyloid-β and tau in Alzheimer's disease
Abstract
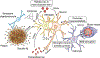
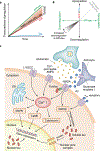
Patients with Alzheimer's disease (AD) present with both extracellular amyloid-β (Aβ) plaques and intracellular tau-containing neurofibrillary tangles in the brain. For many years, the prevailing view of AD pathogenesis has been that changes in Aβ precipitate the disease process and initiate a deleterious cascade involving tau pathology and neurodegeneration. Beyond this 'triggering' function, it has been typically presumed that Aβ and tau act independently and in the absence of specific interaction. However, accumulating evidence now suggests otherwise and contends that both pathologies have synergistic effects. This could not only help explain negative results from anti-Aβ clinical trials but also suggest that trials directed solely at tau may need to be reconsidered. Here, drawing from extensive human and disease model data, we highlight the latest evidence base pertaining to the complex Aβ-tau interaction and underscore its crucial importance to elucidating disease pathogenesis and the design of next-generation AD therapeutic trials.
Conflict of interest statement
Competing interests
The authors declare no competing interests related to this project.
Figures


References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET & Hyman BT Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639 (1992). - PubMed
-
- Ingelsson M et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62, 925–931 (2004). - PubMed
-
- Lleó A, Berezovska O, Growdon JH & Hyman BT Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am. J. Geriatr. Psychiatry 12, 146–156 (2004). - PubMed
-
- Ryan NS et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurol. 15, 1326–1335 (2016). - PubMed
-
- Hyman BT Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch. Neurol. 68, 1062–1064 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

