The role of exome sequencing in newborn screening for inborn errors of metabolism
- PMID: 32778825
- PMCID: PMC8800147
- DOI: 10.1038/s41591-020-0966-5
The role of exome sequencing in newborn screening for inborn errors of metabolism
Abstract
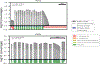
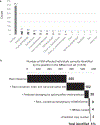
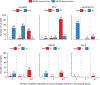
Public health newborn screening (NBS) programs provide population-scale ascertainment of rare, treatable conditions that require urgent intervention. Tandem mass spectrometry (MS/MS) is currently used to screen newborns for a panel of rare inborn errors of metabolism (IEMs)1-4. The NBSeq project evaluated whole-exome sequencing (WES) as an innovative methodology for NBS. We obtained archived residual dried blood spots and data for nearly all IEM cases from the 4.5 million infants born in California between mid-2005 and 2013 and from some infants who screened positive by MS/MS, but were unaffected upon follow-up testing. WES had an overall sensitivity of 88% and specificity of 98.4%, compared to 99.0% and 99.8%, respectively for MS/MS, although effectiveness varied among individual IEMs. Thus, WES alone was insufficiently sensitive or specific to be a primary screen for most NBS IEMs. However, as a secondary test for infants with abnormal MS/MS screens, WES could reduce false-positive results, facilitate timely case resolution and in some instances even suggest more appropriate or specific diagnosis than that initially obtained. This study represents the largest, to date, sequencing effort of an entire population of IEM-affected cases, allowing unbiased assessment of current capabilities of WES as a tool for population screening.
Figures









References
-
- Mak CM, Lee HC, Chan AY & Lam CW Inborn errors of metabolism and expanded newborn screening: review and update. Crit Rev Clin Lab Sci 50, 142–162 (2013). - PubMed
-
- McHugh D, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med 13, 230–254 (2011). - PubMed
-
- Wilcken B, Wiley V, Hammond J & Carpenter K Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med 348, 2304–2312 (2003). - PubMed
-
- Tang H, et al. Damaged goods?: an empirical cohort study of blood specimens collected 12 to 23 hours after birth in newborn screening in California. Genet Med 18, 259–264 (2016). - PubMed
References (Methods only)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

