Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: implications for choline and PUFA metabolism
- PMID: 32778895
- PMCID: PMC7727469
- DOI: 10.1093/ajcn/nqaa207
Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: implications for choline and PUFA metabolism
Abstract
Background: Lipid metabolism in pregnancy delivers PUFAs from maternal liver to the developing fetus. The transition at birth to diets less enriched in PUFA is especially challenging for immature, extremely preterm infants who are typically supported by total parenteral nutrition.
Objective: The aim was to characterize phosphatidylcholine (PC) and choline metabolism in preterm infants and demonstrate the molecular specificity of PC synthesis by the immature preterm liver in vivo.
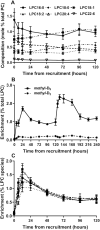
Methods: This MS-based lipidomic study quantified the postnatal adaptations to plasma PC molecular composition in 31 preterm infants <28 weeks' gestational age. Activities of the cytidine diphosphocholine (CDP-choline) and phosphatidylethanolamine-N-methyltransferase (PEMT) pathways for PC synthesis were assessed from incorporations of deuterated methyl-D9-choline chloride.
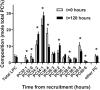
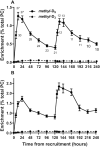
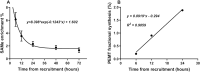
Results: The concentration of plasma PC in these infants increased postnatally from median values of 481 (IQR: 387-798) µM at enrollment to 1046 (IQR: 616-1220) µM 5 d later (P < 0.001). Direct incorporation of methyl-D9-choline demonstrated that this transition was driven by an active CDP-choline pathway that synthesized PC enriched in species containing oleic and linoleic acids. A second infusion of methyl-D9-choline chloride at day 5 clearly indicated continued activity of this pathway. Oxidation of D9-choline through D9-betaine resulted in the transfer of 1 deuterated methyl group to S-adenosylmethionine. A very low subsequent transfer of this labeled methyl group to D3-PC indicated that liver PEMT activity was essentially inactive in these infants.
Conclusions: This study demonstrated that the preterm infant liver soon after birth, and by extension the fetal liver, was metabolically active in lipoprotein metabolism. The low PEMT activity, which is the only pathway for endogenous choline synthesis and is responsible for hormonally regulated export of PUFAs from adult liver, strongly supports increased supplementation of preterm parenteral nutrition with both choline and PUFAs.
Keywords: CDP-choline pathway: PEMT pathway; plasma phosphatidylcholine; preterm infants; stable isotopes.
Copyright © The Author(s) on behalf of the American Society for Nutrition 2020.
Figures



Comment in
-
P4: PEMT, PCs, PUFAs, and prematurity.Am J Clin Nutr. 2020 Dec 10;112(6):1417-1419. doi: 10.1093/ajcn/nqaa270. Am J Clin Nutr. 2020. PMID: 33022706 No abstract available.
References
-
- Doyle LW, Roberts G, Anderson PJ. Changing long-term outcomes for infants 500–999 g birth weight in Victoria, 1979–2005. Arch Dis Child Fetal Neonatal Ed. 2011;96:F443–7. - PubMed
-
- Larque E, Demmelmair H, Gil-Sanchez A, Prieto-Sanchez MT, Blanco JE, Pagan A, Faber FL, Zamora S, Parrilla JJ, Koletzko B. Placental transfer of fatty acids and fetal implications. Am J Clin Nutr. 2011;94(6 Suppl):1908S–13S. - PubMed
-
- Koletzko B, Larque E, Demmelmair H. Placental transfer of long-chain polyunsaturated fatty acids (LC-PUFA). J Perinatal Med. 2007;35(Suppl 1):S5–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

