Correction of serum potassium with sodium zirconium cyclosilicate in Japanese patients with hyperkalemia: a randomized, dose-response, phase 2/3 study
- PMID: 32779057
- PMCID: PMC7599176
- DOI: 10.1007/s10157-020-01937-1
Correction of serum potassium with sodium zirconium cyclosilicate in Japanese patients with hyperkalemia: a randomized, dose-response, phase 2/3 study
Abstract
Background: Sodium zirconium cyclosilicate (SZC) is an oral potassium binder approved to treat hyperkalemia in adults in a number of countries, including Japan.
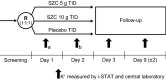
Methods: This phase 2/3, randomized, double-blind, placebo-controlled, dose-response study (ClinicalTrials.gov: NCT03127644) was designed to determine the efficacy and safety of SZC in Japanese adults with hyperkalemia. Patients with serum potassium (sK+) concentrations ≥ 5.1- ≤ 6.5 mmol/L were randomized 1:1:1 to SZC 5 g, SZC 10 g, or placebo three times daily for 48 h (six doses total). The primary efficacy endpoint was the exponential rate of change in sK+ over 48 h. The proportion of patients with normokalemia (sK+ 3.5-5.0 mmol/L) at 48 h and adverse events (AEs) were also evaluated.
Results: Overall, 103 patients (mean age, 73.2 years; range 50-89 years) received SZC 5 g (n = 34), SZC 10 g (n = 36), or placebo (n = 33). The exponential rate of sK+ change from 0 to 48 h versus placebo was - 0.00261 (SZC 5 g) and - 0.00496 (SZC 10 g; both P < 0.0001). At 48 h, the proportions of patients with normokalemia were 85.3%, 91.7%, and 15.2% with SZC 5 g, SZC 10 g, and placebo, respectively. No serious AEs were reported. Hypokalemia (sK+ < 3.5 mmol/L) occurred in two patients in the SZC 10 g group; normokalemia was re-established within 6 days and no treatment-related AEs were reported.
Conclusion: SZC is effective and well tolerated in Japanese patients with hyperkalemia.
Keywords: Hyperkalemia; Japan; Japanese; Sodium zirconium cyclosilicate.
Conflict of interest statement
N Kashihara has received consulting fees from AstraZeneca and has received research grants from AstraZeneca and the Japan Agency for Medical Research and Development. Y Saka, T Nishio, T Osonoi, T Imasawa, T Ohtake, H Mizuno, and Y Shibagaki have no conflicts of interest to disclose. H Kim, T Yajima, and N Sarai are employees of AstraZeneca K.K.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

