Drugs against SARS-CoV-2: What do we know about their mode of action?
- PMID: 32779326
- PMCID: PMC7435512
- DOI: 10.1002/rmv.2143
Drugs against SARS-CoV-2: What do we know about their mode of action?
Abstract
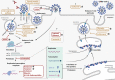
The health emergency caused by the recent Covid-19 pandemic highlights the need to identify effective treatments against the virus causing this disease (SARS-CoV-2). The first clinical trials have been testing repurposed drugs that show promising anti-SARS-CoV-2 effects in cultured cells. Although more than 2400 clinical trials are already under way, the actual number of tested compounds is still limited to approximately 20, alone or in combination. In addition, knowledge on their mode of action (MoA) is currently insufficient. Their first results reveal some inconsistencies and contradictory results and suggest that cohort size and quality of the control arm are two key issues for obtaining rigorous and conclusive results. Moreover, the observed discrepancies might also result from differences in the clinical inclusion criteria, including the possibility of early treatment that may be essential for therapy efficacy in patients with Covid-19. Importantly, efforts should also be made to test new compounds with a documented MoA against SARS-CoV-2 in clinical trials. Successful treatment will probably be based on multitherapies with antiviral compounds that target different steps of the virus life cycle. Moreover, a multidisciplinary approach that combines artificial intelligence, compound docking, and robust in vitro and in vivo assays will accelerate the development of new antiviral molecules. Finally, large retrospective studies on hospitalized patients are needed to evaluate the different treatments with robust statistical tools and to identify the best treatment for each Covid-19 stage. This review describes different candidate antiviral strategies for Covid-19, by focusing on their mechanism of action.
© 2020 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors have no competing interest.
Figures
References
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953‐1966. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, RAM F. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

