Loss of supervillin causes myopathy with myofibrillar disorganization and autophagic vacuoles
- PMID: 32779703
- PMCID: PMC7447519
- DOI: 10.1093/brain/awaa206
Loss of supervillin causes myopathy with myofibrillar disorganization and autophagic vacuoles
Erratum in
-
Corrigendum to: Loss of supervillin causes myopathy with myofibrillar disorganization and autophagic vacuoles.Brain. 2021 Apr 12;144(3):e34. doi: 10.1093/brain/awaa412. Brain. 2021. PMID: 33313686 Free PMC article. No abstract available.
Abstract
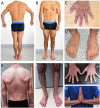
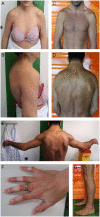
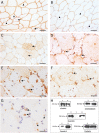
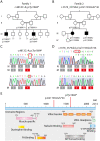
The muscle specific isoform of the supervillin protein (SV2), encoded by the SVIL gene, is a large sarcolemmal myosin II- and F-actin-binding protein. Supervillin (SV2) binds and co-localizes with costameric dystrophin and binds nebulin, potentially attaching the sarcolemma to myofibrillar Z-lines. Despite its important role in muscle cell physiology suggested by various in vitro studies, there are so far no reports of any human disease caused by SVIL mutations. We here report four patients from two unrelated, consanguineous families with a childhood/adolescence onset of a myopathy associated with homozygous loss-of-function mutations in SVIL. Wide neck, anteverted shoulders and prominent trapezius muscles together with variable contractures were characteristic features. All patients showed increased levels of serum creatine kinase but no or minor muscle weakness. Mild cardiac manifestations were observed. Muscle biopsies showed complete loss of large supervillin isoforms in muscle fibres by western blot and immunohistochemical analyses. Light and electron microscopic investigations revealed a structural myopathy with numerous lobulated muscle fibres and considerable myofibrillar alterations with a coarse and irregular intermyofibrillar network. Autophagic vacuoles, as well as frequent and extensive deposits of lipoproteins, including immature lipofuscin, were observed. Several sarcolemma-associated proteins, including dystrophin and sarcoglycans, were partially mis-localized. The results demonstrate the importance of the supervillin (SV2) protein for the structural integrity of muscle fibres in humans and show that recessive loss-of-function mutations in SVIL cause a distinctive and novel myopathy.
Keywords: SVIL; cardiac disease; costameric protein; myopathy; supervillin.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Chen Y, Takizawa N, Crowley JL, Oh SW, Gatto CL, Kambara T, et al.F-actin and myosin II binding domains in supervillin. J Biol Chem 2003; 278: 46094–106. - PubMed
-
- Chen X, Yang H, Zhang S, Wang Z, Ye F, Liang C, et al.A novel splice variant of supervillin, SV5, promotes carcinoma cell proliferation and cell migration. Biochem Biophys Res Commun 2017; 482: 43–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

