Dependence of PINK1 accumulation on mitochondrial redox system
- PMID: 32779864
- PMCID: PMC7511888
- DOI: 10.1111/acel.13211
Dependence of PINK1 accumulation on mitochondrial redox system
Abstract
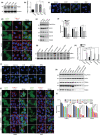
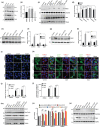
Accumulation of PINK1 on the outer mitochondrial membrane (OMM) is necessary for PINK-mediated mitophagy. The proton ionophores, like carbonyl cyanide m-chlorophenylhydrazone (CCCP) and carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), inhibit PINK1 import into mitochondrial matrix and induce PINK1 OMM accumulation. Here, we show that the CHCHD4/GFER disulfide relay system in the mitochondrial intermembrane space (IMS) is required for PINK1 stabilization when mitochondrial membrane potential is lost. Activation of CHCHD4/GFER system by mitochondrial oxidative stress or inhibition of CHCHD4/GFER system with antioxidants can promote or suppress PINK1 accumulation, respectively. Thus data suggest a pivotal role of CHCHD4/GFER system in PINK1 accumulation. The amyotrophic lateral sclerosis-related superoxide dismutase 1 mutants dysregulated redox state and CHCHD4/GFER system in the IMS, leading to inhibitions of PINK1 accumulation and mitophagy. Thus, the redox system in the IMS is involved in PINK1 accumulation and damaged mitochondrial clearance, which may play roles in mitochondrial dysfunction-related neurodegenerative diseases.
Keywords: PINK1; autophagy; mitochondrion; mitophagy; neurodegenerative diseases.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no competing financial interest.
Figures



References
-
- Chacinska, A. , Pfannschmidt, S. , Wiedemann, N. , Kozjak, V. , Sanjuán Szklarz, L. K. , Schulze‐Specking, A. , … Pfanner, N. (2004). Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO Journal, 23(19), 3735–3746. 10.1038/sj.emboj.7600389 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

